On the hidden significance of differing micro-sites on tree-ring based climate reconstructions
Düthorn E., Schneider L., Konter O., Schön P., Timonen M., Esper J. (2015). On the hidden significance of differing micro-sites on tree-ring based climate reconstructions. Silva Fennica vol. 49 no. 1 article id 1220. https://doi.org/10.14214/sf.1220
Highlights
- Pines and spruces show growth level differences in wet and dry micro-sites with higher growth rates in the dry sites
- Spruces show a robust climate-growth relationship with June-July temperatures
- Application of collective detrending methods can bias long-term trends in climate reconstructions, if relict and recent samples originate from different micro-sites.
Abstract
Tree-ring chronologies are commonly extended back in time by combining samples from living trees with relict material preserved in man-made structures or natural archives (e.g. lakes). Although spatially close, these natural archives and living-tree-sites often comprise different micro-climates. Inhomogeneous growth conditions among these habitats, which may yield offsets in growth-rates, require caution in data processing. Here we assess species-specific growth dynamics in two micro-habitats and their potential effects on long chronologies by combining tree-ring data from different living-tree-sites with an “artificial” subfossil dataset. Well replicated (n > 80) Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris L.) chronologies from northern Fennoscandia, sampled directly at the lakeshore (wet) and several meters beyond the lakeshore (dry) reveal high coherence of the variance between micro-sites (rspruce = 0.59, rpine = 0.68). Significant differences of the Regional Curves (RC) indicate faster growth of both species at the drier site though. Growth differences are more pronounced between the spruce micro-sites. The combination of recent dry and wet spruce data with artificial relict data results in two long chronologies covering the last 800 years with substantially different trends, although they consist of the same relict material and the micro-site chronologies correlate significantly over the past two centuries. The combination of spruce samples from dry inland micro-sites with subfossil samples originating from the wet lake shore can result in an underestimation of past temperatures prior to the 19th century. Such effects, hidden in the composition of long chronologies (living trees + subfossil samples) can bias long-term trends in climate reconstructions.
Keywords
Pinus sylvestris;
climate change;
Picea abies;
Finland;
temperature reconstruction;
RCS detrending
-
Düthorn,
Department of Geography, Johannes Gutenberg University, Becherweg 21, 55099 Mainz, Germany
E-mail
duethorn@uni-mainz.de

- Schneider, Department of Geography, Johannes Gutenberg University, Becherweg 21, 55099 Mainz, Germany E-mail l.schneider@geo.uni-mainz.de
- Konter, Department of Geography, Johannes Gutenberg University, Becherweg 21, 55099 Mainz, Germany E-mail O.Konter@geo.uni-mainz.de
- Schön, Department of Geography, Johannes Gutenberg University, Becherweg 21, 55099 Mainz, Germany E-mail philipp.schoen@gmx.de
- Timonen, Natural Resources Institute Finland (Luke), Natural resources and bioproduction, FI-96301 Rovaniemi, Finland E-mail mauri.timonen@metla.fi
- Esper, Department of Geography, Johannes Gutenberg University, Becherweg 21, 55099 Mainz, Germany E-mail J.Esper@geo.uni-mainz.de
Received 7 July 2014 Accepted 27 January 2015 Published 5 February 2015
Views 124551
Available at https://doi.org/10.14214/sf.1220 | Download PDF

1 Introduction
Tree rings are a common proxy to reconstruct past climate variations. At extreme locations, where climate determines tree growth, common variations in tree ring width (TRW) are observed and allow reconstructions of growing-season temperature history, especially referring to TRW measurements from high altitudinal or latitudinal sites (Fritts 1976; Grudd et al. 2002; Helama et al. 2002; Büntgen et al. 2006). In northern Fennoscandia the main species used for such dendrochronological and dendroclimatological studies is Scots pine (Pinus sylvestris L.) (Schweingruber et al. 1988; Eronen et al. 2002; Grudd et al. 2002; Büntgen et al. 2011; Gunnarson et al. 2011; Esper et al. 2012; Melvin et al. 2013). Next to the pines also spruces grow in Northern Fennoscandia although their ecological limit does not extend as far north (Skrøppa 2003). Some spruce populations (Picea abies (L.) Karst.) are also included in tree-ring analyses and provide information about past summer temperatures (Gouirand et al. 2008; Büntgen et al. 2011).
In order to extend climate reconstructions beyond maximum tree-age, tree-ring chronologies are complemented with dead wood material (herein “relict material”) preserved, for instance, in the numerous lakes of northern Fennoscandia (herein “subfossil material”). Overlapping life spans of these logs with recent material and homogeneous growth patterns, caused by common limiting factors, enable annual dating and combination of these samples with living trees (Fritts 1976). The Regional Curve Standardization (RCS) requires equivalent provenances of dead and living wood samples representing the same ecological and climate setting. Confining such homogeneous sampling sites, however, remains ambiguous. Düthorn et al. (2013) showed that even spatially close populations of Pinus sylvestris might comprise different micro-habitats which affect growth-rates and -patterns.
Micro-site effects are not only related to soil moisture content. Similar problems in the standardization process develop when combining samples from different elevations. In Gunnarson et al. (2011) a difference in altitude leads to variations in growth rates that need to be adjusted before the data are combined to mean chronologies. Similar limitations have been addressed in Melvin et al. (2013) when connecting relict and living material and updating the density datasets of Schweingruber (Schweingruber et al. 1988) and Grudd (Grudd 2008). The differences described in Melvin et al. (2013) are not only site related but also due to different methods of maximum latewood density measurements (Esper et al. 2014). Differing growth levels require a separate detrending for each dataset if a common detrending is applied. However, this multiple RCS approach results in a loss of low-frequency climate variability (Briffa and Melvin 2011). Another approach in achieving comparability is made by Tegel et al. (2010). Relict material of heterogeneous origin should not be combined with recent samples from a homogeneous site. Thus, they suggest to select the wood of the living part randomly, e.g. in sawmills, to assure heterogeneity in both datasets. The homogeneity of the different wood sources should be tested using the period of overlap.
Here, we analyze well replicated micro-sites of pines and spruces from northern Finland. Site and species related characteristics are identified by focusing on growth rates and climate sensitivity. In addition, the influence of collective detrending methods on compiled chronologies and temperature reconstructions is tested and discussed with reference to consequences of hidden ecological effects and common pitfalls for TRW-standardization (Esper et al. 2005b, Briffa and Melvin 2011). These effects could have influence on the longer term assessment of climate change with paleoclimate records. Our study detects this hidden statistical bias and details ideas to avoid developing erroneous time series.
2 Material and methods
2.1 Tree-ring data
The study area is located at 68.45°N and 27.36°E in northern Finland (Fig. 1). The sampling design differentiates between trees growing directly at the lakeshore (Fig. 1: “wet” ) and trees standing a few meters beyond the lakeshore (Fig. 1: “dry”) (Düthorn et al. 2013). The dataset consists of 50 Picea abies and 50 Pinus sylvestris per micro-site (spruce_wet/spruce_dry and pine_wet/pine_dry, respectively). After crossdating all tree-ring time series, measured to an accuracy of 1/100mm, we applied a Regional Curve Standardization (RCS) to remove biological age trends while keeping low-frequency information (Briffa et al. 1992; Esper et al. 2003). This method generates a mean growth curve by aligning all TRW-series by cambial age. This “Regional Curve” (RC) represents the local growth behavior and displays the mean age trend of tree growth at a certain site. By dividing every single TRW-series by this RC the biological age effect is removed.
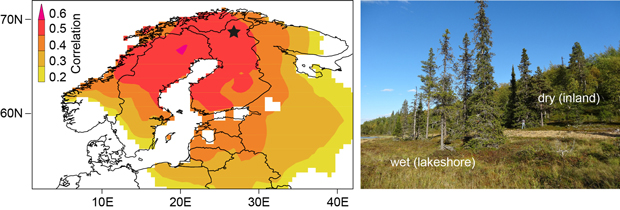
Fig. 1. Sampling area in northern Finland (black star) with the spatial correlation of the spruce chronology with gridded June-July temperatures (CRU TS 3.10, p < 5%) over the 1931–2009 period. The picture on the right side shows the micro-site sampling design. Trees growing at the lakeshore (on the inland slope) with more moist (dry) soil conditions are termed “wet” (“dry”).
2.2 Climate data
Climate-growth relationships are assessed for different climate parameters. We used the 0.5° × 0.5° CRU TS 3.10 dataset for comparison with precipitation averaged over the 66–70°N and 25–30°E grid-cells (Mitchell and Jones 2005). Over the same area a snow index, based on surface color, is extracted from the Rutgers University Global Snow Lab (Brown et al. 2003). Monthly temperature data for the 20th century are derived from the nearby station Sodankylä. The common period 1931–2011 only covers 81 years since old trees were absent at one of the microsites. The period is defined by the maximum overlap of the micro-site chronologies with a minimum replication of 5 series. For large-scale temperature analysis, the tree ring chronologies were correlated against mean June-July (JJ) values of various grid cells over northern Europe.
2.3 Artificial relict data
A millennial dataset of spruce TRW is not available for northern Fennoscandia. Thus, artificial data had to be created for assessing effects of micro-sites on long-term spruce chronologies. We adjusted the relict part of the subalpine spruce chronology of Büntgen et al. (2005) to match the properties of a lakeshore site in northern Fennoscandia corresponding to the original habitat of submerged logs. The distant setting of the subalpine relict material results in a mismatch of annual variation in the period of overlap with the fennoscandian living datasets. But more important in assessing a potential long-term bias is to retain the species-specific age-related growth trends. Therefore, we synchronized the regional curve of the subalpine relict spruce data to the spruce_wet dataset by removing the original age trend of the alpine tree ring series by applying RCS detrending. The detrended single series were multiplied with the mean growth rate of the spruce_wet site (Fig. 5) assuming that trees at the lakeshore show equal mean growth curves like subfossil logs (Düthorn et al. 2013). Consequently, the mean growth rate of the subalpine chronology is similar to the mean growth rate of the lakeshore spruce site. Finally, living and relict material was combined to two long-term spruce chronologies with different recent tails (wet and dry).
3 Results
3.1 Variability of micro-site chronologies
Both, wet and dry RCS chronologies display the similar patterns in high and low frequency variations (Fig. 2) and correlate at r = 0.59 (spruce) and r = 0.68 (pine) for the last 81 years. The consistent shape of these micro-site chronologies shows that annual variations in tree growth at both stands are controlled by a common external driver.
RCS creates a chronology whose mean value is approximately 1.0 and separately processing micro sites with RCS will remove any between-site differences in the mean growth rates of trees. The resulting spruce and pine chronologies allow inter-species comparisons and reveal disparities in growth on long and short terms (Fig. 2c, r = –0.2). Especially the drop of the pine chronology in the beginning of the 20th century indicates extreme species-specific growth-disturbance. After this drop and a subsequent steep increase of growth, the trend for the last 90 years is slightly decreasing while the spruces are much more complacent in their low-frequency signal. Both species exhibit an unequal age structure and replication. While the number of spruce trees declines linearly before AD 2000, there are two major steps in the pine chronology replication (1940–1950 and 1980–1990; Fig. 2d) resulting in a lower mean age of pine individuals in the second half of the 20th century.
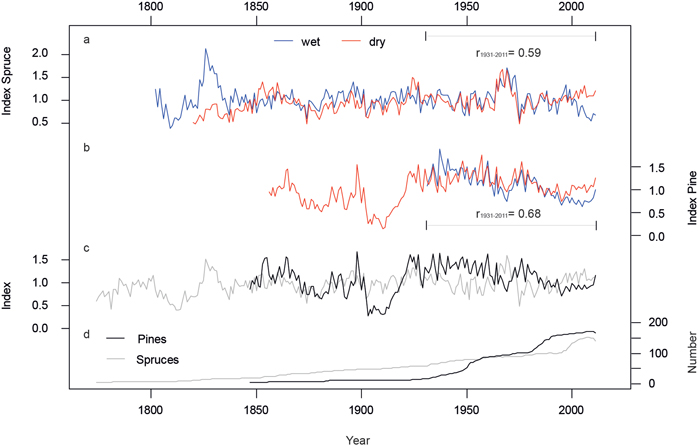
Fig. 2. Micro-site and species specific chronologies after Regional Curve Standardization. a) Spruce chronologies from the wet (blue) and dry (red) micro-sites. b) Same as in a) but for pine. c) Pine (black) and spruce (grey) site chronologies, each including wet and dry trees. d) Numbers of measurement series averaged in the site chronologies. All chronologies were truncated at n < 4. View larger in new window/tab.
3.2 Climate growth relationships
The response of the trees to climate is displayed by the high correlation for spruce TRW chronologies with summer temperatures (Fig. 3).
Monthly temperature responses are calculated for the previous and current year using temperature data from Sodankylä over the 1931–2011 common period (Fig. 3). Strongest climate-growth relationships for spruces on a monthly basis are detected for June (r = 0.49), while the highest seasonal response is found in JJ (r = 0.55) and JJA (r = 0.54). Pines only correlate insignificantly (r = 0.30) with temperatures in July (Fig. 3), which is also the month of highest wood production (Schmitt et al. 2004). Also the spruce micro-site chronologies reveal robust June temperature signals, whereas data from both pine micro-sites display slightly negative correlation values in June. Other climate parameters, e.g. precipitation and snow cover, do not show significant influences on microsite- or species-related tree growth.
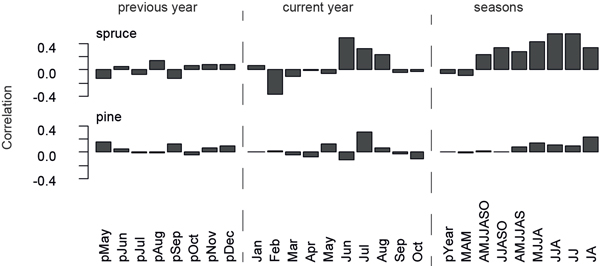
Fig. 3. Correlation of spruce and pine chronologies with temperature data recorded at Sodankylä over the 1931–2011 common period. Correlations are calculated for previous year and current year months, as well as seasonal temperature data.
The impact of the previous year temperature on the current year growth is a well-known feature of TRW data (Briffa et al. 1988; Kozlowski and Pallardy 1996). This memory effect is displayed by strong lag-1 autocorrelations for the chronologies (pine: ac1931–2011 = 0.61, spruce: ac1931–2011 = 0.48).
The differences in the monthly climate response of both species indicates characteristic species-related biological and ecological demands. Fig. 4 shows the regional climograph combining temperature, precipitation and the snow cover index. Between May and October the mean temperature is above 0°C while only the summer temperatures (June-September) rise above the physiologically important threshold of 5 °C (Körner 2012) with simultaneously peaking precipitation values, defining the vegetation period. At high latitudes a full snow-cover from November to May is the key constraint for vegetation (Vaganov et al. 1999). In June and October a light snow cover is detected while for the period July-August-September (JAS) no snow is reported. The strong decrease of the snow index between May and June is an indicator for high amounts of melt water, introducing very wet conditions in early summer.
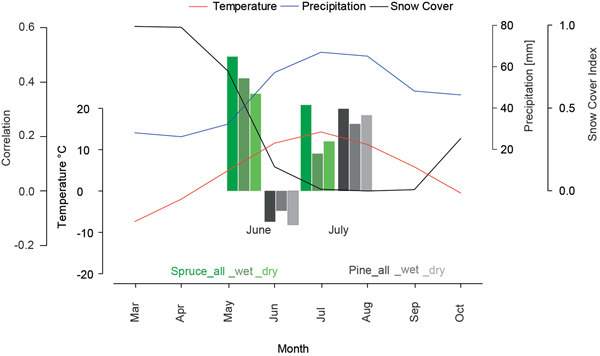
Fig. 4. Monthly mean temperature (red), precipitation (blue) and snow cover (black) for northern Fennoscandia over the 1971–2000 period. Barplot represents correlations of different tree-ring chronologies (site and micro-sites) over the 1931–2011 period with June and July temperatures.
3.3 Growth rate differences
Micro-site effects in pine chronologies are already recognized (Düthorn et al. 2013) but the ecological effects for spruces are unknown so far. Fig. 5 shows the Regional Curves for the wet and dry spruce sites (wet_RC/dry_RC) and a distinct offset between both curves for the first 200 years of growth. At the dry site mean annual increment exceeds 1.5 mm for the first 50 years and declines afterwards linearly to an annual increment of 0.6 mm after 200 years of growth. At the wet site growth does not exceed 1 mm/year, and after 200 years it reaches a mean growth of only 0.4mm/year. We assume that wet, anaerobic soil conditions limit tree growth especially in the juvenile growth phase. This effect is confirmed by the RC’s of the pines analyzed in this study, where also the wet micro-site shows less wood production compared to the dry site. The flat wet_RC mimics characteristics of suppressed growth in closed canopy stands (Helama et al. 2005).
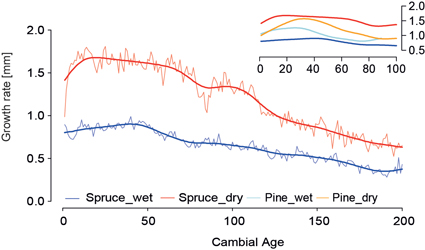
Fig. 5. Age-aligned regional curves and 40-year smoothed mean curves (thick lines) of the spruce micro-site chronologies. Blue (red) represents lakeshore (inland) trees. Regional curves are shown over the first 200 years. Upper panel shows smoothed regional curves of spruce and pine micro-site chronologies for the first 100 years.
3.4 Combined chronologies and tail test
For tests on long-term trends, TRW series from the adjusted “subfossil” data were combined with either TRW series from the wet or dry micro-sites. We applied RCS to both combined datasets (subfossil + wet and subfossil + dry). The overlapping period of relict and living TRW data (Fig. 6d) is defined by the start of the living spruce chronology (1695) and the last year with data of the relict part (1833). During these 139 years of overlap variance is reduced since growth variations are not coherent for the alpine and fennoscandian region. However, multi-centennial trends bridge this period and are preserved. The final dataset covers the last eight centuries (1163–2011) with a minimum replication of 6 series. The climate-growth relationship of spruce and JJ temperature (rspruce = 0.54) was used to calculate a linear regression model for both long-term chronologies. Finally, the tree-ring indices were transformed into JJ temperature anomalies with respect to the 1961–1990 period (Fig. 6a and 6b).
Both reconstructions show synchronized variations and mean values for the most recent period back to 1800. Prior to 1800 the low-frequency trends are very similar but a peculiar level offset is obvious between Fig. 6 a and b. The wet_reconstruction indicates colder (14th and 17th century) and warmer (end of the 13th century and 16th century) periods compared to the 1961–1990 period. The differences vary between +1 °C and –1 °C. In contrast, the dry_reconstruction generally points to colder conditions prior to 1800, fluctuating around –2 °C except for the late 13th century. By scaling both reconstructions over the early period (1163–1694, dashed line in Fig. 6c) the high coherency between both time series becomes obvious whereas for the most recent period a distinct level offset appears. No millennial cooling trend is retained in the dry_reconstruction, whereas the wet_reconstruction reveals slightly such a trend (thick dotted line in Fig. 6a and Fig. 6b). These differences can be traced back to the higher growth rates in dry stands which are not sufficiently reflected in the RC and thus not eliminated after detrending.
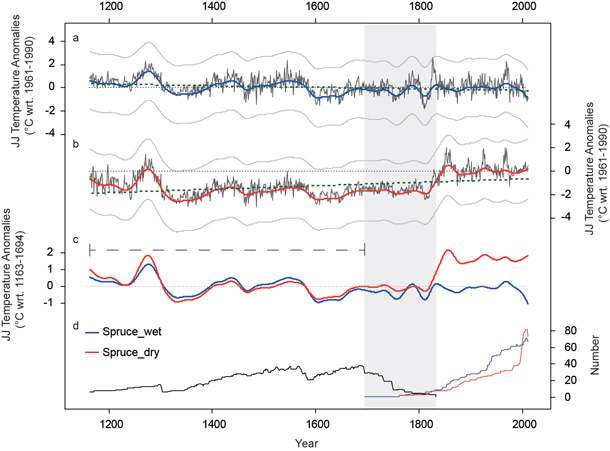
Fig. 6. JJ temperature reconstructions based on long-term spruce chronologies (black). a) Lakeshore wood material (wet) and b) inland (dry) material is used for the living part. The reconstruction extents back to 1163 AD. Low frequency variability of decadal to centennial timescale is displayed by colored lines (50 year spline filter). Green dotted lines show the trend over the last 8 centuries. Uncertainties (+/- standard error) are displayed as error band (grey lines). Thin dotted lines are the 0 °C anomaly level. c) Smoothed JJ temperature reconstructions scaled over the 1163–1694 common period (only relict material; black dashed line). d) Replication of relict (black), living_wet (blue) and living_dry (red) tree ring series. Grey area displays the maximum overlap (1695–1833 period) of the different wood sources.
4 Discussion
Tree growth in higher latitudes is directly dependent on the ecological setting at the micro-sites. Independent of the tree species, growth rates are lower for trees growing at the lakeshore. Our results indicate that tree growth at lakeshores is limited and suppressed. Düthorn et al. (2013) assumed that this effect is linked to a high groundwater table and hence anaerobic soil conditions. This effect is stronger for spruce compared to pine.
After removing site-specific growth characteristics spruce and pine chronologies from northern Finland show high intra-species homogeneity resulting from a common and site-independent sensitivity to external factors (i.e. summer temperature for spruce). In contrast to the intra-species homogeneity, no inter-species agreement can be detected. The inter-species discrepancy may indicate that spruce and pine respond different to external influences. But as displayed in Büntgen et al. (2011) and Gouirand et al. (2008) both species are principally sensitive to summer temperatures in these latitudes.
In this study we found a higher sensitivity to summer temperatures for spruces and a weaker response in pines. Our study area is located 8 km southwest from a pine population in Laanila from which a significant and temporally robust summer temperature signal (r = 0.50) is reported for TRW (McCarroll et al. 2003). Low correlations with temperature despite related growth patterns among wet and dry sites indicate a more complex growth response at our site. The site-related disturbances, either of climatic, anthropogenic or ecological origin, affect the temperature sensitivity of the pines.
Therefore, the differences need to be associated with differing ecological needs of the species. The indistinct temperature response of pines suggests that this species cannot deal with the moist soil conditions caused by high precipitation sums and melt water runoff and hence sprouts out later when temperatures are higher and soils drier. Spruces in contrast, start growing as early as temperatures rise over the 5 °C threshold in June. This species can also deal with light snow cover and sprouts earlier leading to a prolonged vegetation period as expressed in the correlations of the micro-site chronologies against June and July temperatures (barplot in Fig. 4). Therefore pines are most sensitive to temperatures in July while spruces already respond to June temperatures. Spruces also show a robust temperature signal spanning from June to July and thereby offering the opportunity to retain information about past early growing season temperatures.
The establishment of a long-term TRW or density chronology based on subfossil spruces is hindered by the natural process of wood decomposition. The lower content of resin compared to pines results in a poor conservation and preservation of tree trunks in lakes or lake sediments. The use of “artificial” data for the relict part allows us to point to the effects that micro-sites could have on tree ring chronologies but not to compare it with existing temperature reconstructions of this region, as Torneträsk (Grudd et al. 2002) or Nscan (Esper et al. 2012). In order to demonstrate that the micro-habitat of living trees matters if recent material is combined with relict wood, we calculated two reconstructions with different recent ends (“wet” and “dry”). The hypothesis that lakeshore trees comprise the same ecological information as subfossil trees, formerly standing at the lakeshore, justifies the assumption that growth rates for the “artificial” part should mimic growth rates of living spruces from the lakeshore. In other words, the sampling design for a living tree site should aim at a close representation of the ecological setting for relict trees in order to avoid problems when collective detrending methods are applied (see “Tail Test”; Düthorn et al. (2013)).
Despite the homogeneity of the short micro-site chronologies the long reconstructions diverge in the most recent part. Although this effect could be misinterpreted as a “hidden” information contained in the micro-sites, it is basically only the result of faster tree growth at the inland site. As RCS is a collective detrending method relict and recent tree ring series were standardized by dividing every single raw series through the same mean growth rate. But the division by an undersized RC for trees from the dry micro-site (wet < dry) and an overestimated growth rate for the relict material creates biases in the chronologies’ long term trend reconstructions. In conclusion, the divergence of the temperature reconstructions could be traced back to different growth behavior according to micro-habitats in combination with RCS detrending.
In order to avoid the propagation of these effects into multi-centennial climate-reconstructions, where they can obscure long-term trends (Esper et al. 2012), a closer look at the sampled material is necessary. The problem of combining living and historical material is also reported in various studies (Tegel et al. 2010; Gunnarson et al. 2011; Melvin et al. 2013) showing that this “hidden” micro-site effect is important in connection with updating and improving long-term chronologies. Beside the effects of sampling strategies on mean chronologies (Nehrbass-Ahles et al. 2014) special care is needed in the selection of statistical methods used to transform tree-ring data into climate reconstructions (Esper et al. 2005b, Esper et al. 2005a, Moberg et al. 2006).
Our study contributes a micro-site assessment for a new species to the existing studies and should help to avoid common pitfalls in developing a long term spruce chronology. Also a higher comparability of the climate signal stored in the trees of the same micro habitat (relict vs. wet) should increase the reliability of long term tree ring chronologies.
5 Conclusion
Millennial-long summer temperature reconstructions are a valuable product of dendroclimatology. Their development, however, requires a thorough assessment of data characteristics and methods as proven by a number of recent studies (Düthorn et al. 2013; Nehrbass-Ahles et al. 2014). This extended micro-site study in northern Finland is designed to expose potential pitfalls in sampling-site selection. Along a lake-inland gradient tree-growth of spruces and pines differs significantly regarding mean annual increment and climate sensitivity. The climate-growth relationship between pines and July temperatures is weak, whereas spruces show a robust June-July signal. The growth rates of both species indicate that lakeshore trees grow significantly slower than trees in the inland do. Even though the micro-site chronologies themselves show high coherence, this site-related effect is responsible for offsets in the mean of long-term chronologies compiled of relict and recent tree-samples, mainly when wood from different ecological provenances is used. Micro-site ecology matters even more for spruce trees than for pines. Consequently, the hidden micro-site effect constrains the spatial comparability of ‘Regional’ Curves and requires species-independent caution in the development of temperature reconstructions based on a collective detrending method. Due to the importance of RCS detrending regarding the preservation of low-frequency information, it is necessary to analyze the ecological setting and growth behavior of subsets of wood samples if it is intended to combine these into a single chronology. Next to the recommendations for future studies this effect should also be taken into account while interpreting existing temperature reconstructions.
This study assumes that subfossil wood has a similar growth rate as living lakeshore trees and uses this hypothesis for simulating “artificial” data. Good preserved relict spruce material would offer new possibilities to interpret micro-site effects on long term spruce chronologies.
Acknowledgements
Supported by the Mainz Geocycles Research Centre.
References
Briffa K.R., Jones P.D., Bartholin T.S., Eckstein D., Schweingruber F.H., Karlen W., Zetterberg P., Eronen M. (1992). Fennoscandian summers from AD-500 – temperature-changes on short and long timescales. Climate Dynamics 7: 111–119. http://dx.doi.org/10.1007/BF00211153.
Briffa K.R., Jones P.D., Pilcher J.R., Hughes M.K. (1988). Reconstructing summer temperatures in northern Fennoscandinavia back to A.D. 1700 using tree-ring data from Scots pine. Arctic and Alpine Research 20: 385–394. http://dx.doi.org/10.2307/1551336.
Briffa K.R., Melvin T.M. (2011). A closer look at regional curve standardization of tree-ring records: justification of the need, a warning of some pitfalls, and suggested improvements in its application. In: Hughes M.K., Swetnam T.W., Diaz H.F. (eds.). Dendroclimatology. Springer Netherlands. p. 113–145. http://dx.doi.org/10.1007/978-1-4020-5725-0_5.
Brown R.D., Brasnett B., Robinson D. (2003). Gridded North American monthly snow depth and snow water equivalent for GCM evaluation. Atmosphere-Ocean 41: 1–14. http://dx.doi.org/10.3137/ao.410101.
Büntgen U., Frank D.C., Schmidhalter M., Neuwirth B., Seifert M., Esper J. (2005). Growth/climate response shift in a long subalpine spruce chronology. Trees – Structure and Function 20: 99–110.
Büntgen U., Bellwald I., Kalbermatten H., Schmidhalter M., Frank D.C., Freund H., Bellwald W., Neuwirth B. et al. (2006). 700 years of settlement and building history in the Lotschental, Switzerland. Erdkunde 60: 96–112. http://dx.doi.org/10.3112/erdkunde.2006.02.02.
Büntgen U., Raible C.C., Frank D., Helama S., Cunningham L., Hofer D., Nievergelt D., Verstege A. et al. (2011). Causes and consequences of past and projected Scandinavian summer temperatures, 500–2100 AD. Plos One 6. http://dx.doi.org/10.1371/journal.pone.0025133.
Düthorn E., Holzkämper S., Timonen M., Esper J. (2013). Influence of micro-site conditions on tree-ring climate signals and trends in central and northern Sweden. Trees 27: 1395–1404. http://dx.doi.org/10.1007/s00468-013-0887-8.
Eronen M., Zetterberg P., Briffa K.R., Lindholm M., Merilainen J., Timonen M. (2002). The supra-long Scots pine tree-ring record for Finnish Lapland: Part 1, chronology construction and initial inferences. Holocene 12: 673–680. http://dx.doi.org/10.1191/0959683602hl580rp.
Esper J., Cook E.R., Krusic P.J., Peters K., Schweingruber F.H. (2003). Tests of the RCS method for preserving low-frequency variability in long tree-ring chronologies. Tree-Ring Research 59: 81–98.
Esper J., Frank D.C., Wilson R.J.S., Briffa K.R. (2005a). Effect of scaling and regression on reconstructed temperature amplitude for the past millennium. Geophysical Research Letters 32.
Esper J., Wilson R.J.S., Frank D.C., Moberg A., Wanner H., Luterbacher J. (2005b). Climate: past ranges and future changes. Quaternary Science Reviews 24: 2164–2166. http://dx.doi.org/10.1016/j.quascirev.2005.07.001.
Esper J., Frank D.C., Timonen M., Zorita E., Wilson R.J.S., Luterbacher J., Holzkamper S., Fischer N. et al. (2012). Orbital forcing of tree-ring data. Nature Climate Change 2: 862–866. http://dx.doi.org/10.1038/nclimate1589.
Esper J., Düthorn E., Krusic P.J., Timonen M., Büntgen U. (2014). Northern European summer temperature variations over the Common Era from integrated tree-ring density records. Journal of Quaternary Science 29(5): 487–494. http://dx.doi.org/10.1002/jqs.2726.
Fritts H.C. (1976). Tree rings and climate. Academic Press. 567 p.
Gouirand I., Linderholm H.W., Moberg A., Wohlfarth B. (2008). On the spatiotemporal characteristics of Fennoscandian tree-ring based summer temperature reconstructions. Theoretical and Applied Climatology 91: 1–25. http://dx.doi.org/10.1007/s00704-007-0311-7.
Grudd H. (2008). Tornetrask tree-ring width and density AD 500–2004: a test of climatic sensitivity and a new 1500-year reconstruction of north Fennoscandian summers. Climate Dynamics 31: 843–857. http://dx.doi.org/10.1007/s00382-007-0358-2.
Grudd H., Briffa K.R., Karlen W., Bartholin T.S., Jones P.D., Kromer B. (2002). A 7400-year tree-ring chronology in northern Swedish Lapland: natural climatic variability expressed on annual to millennial timescales. Holocene 12: 657–665. http://dx.doi.org/10.1191/0959683602hl578rp.
Gunnarson B.E., Linderholm H.W., Moberg A. (2011). Improving a tree-ring reconstruction from west-central Scandinavia: 900 years of warm-season temperatures. Climate Dynamics 36: 97–108. http://dx.doi.org/10.1007/s00382-010-0783-5.
Helama S., Lindholm M., Timonen M., Merilainen J., Eronen M. (2002). The supra-long Scots pine tree-ring record for Finnish Lapland: Part 2, interannual to centennial variability in summer temperatures for 7500 years. Holocene 12: 681–687. http://dx.doi.org/10.1191/0959683602hl581rp.
Helama S., Timonen M., Lindholm M., Merilainen J., Eronen M. (2005). Extracting long-period climate fluctuations from tree-ring chronologies over timescales of centuries to millennia. International Journal of Climatology 25: 1767–1779. http://dx.doi.org/10.1002/joc.1215.
Körner C. (2012). Alpine treelines. Springer. 220 p. http://dx.doi.org/10.1007/978-3-0348-0396-0.
Kozlowski T.T., Pallardy S.G. (1996). Physiology of woody plants. Elsevier Science.
McCarroll D., Jalkanen R., Hicks S., Tuovinen M., Gagen M., Pawellek F., Eckstein D., Schmitt U. et al. (2003). Multiproxy dendroclimatology: a pilot study in northern Finland. Holocene 13: 829–838. http://dx.doi.org/10.1191/0959683603hl668rp.
Melvin T.M., Grudd H., Briffa K.R. (2013). Potential bias in ‘updating’ tree-ring chronologies using regional curve standardisation: re-processing 1500 years of Tornetrask density and ring-width data. Holocene 23: 364–373. http://dx.doi.org/10.1177/0959683612460791.
Mitchell T.D., Jones P.D. (2005). An improved method of constructing a database of monthly climate observations and associated high-resolution grids. International Journal of Climatology 25: 693–712. http://dx.doi.org/10.1002/joc.1181.
Moberg A., Sonechkin D.M., Holmgren K., Datsenko N.M., Karlen W., Lauritzen S.E. (2006). Corrigendum: Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data. [Correction to Nature 433: 613–617, 2005]. Nature 439: 1014. http://dx.doi.org/10.1038/nature04575.
Nehrbass-Ahles C., Babst F., Klesse S., Nötzli M., Bouriaud O., Neukom R., Dobbertin M., Frank D. (2014). The influence of sampling design on tree-ring based quantification of forest growth. Global Change Biology 20(9): 2867–2885. http://dx.doi.org/10.1111/gcb.12599.
Schmitt U., Jalkanen R., Eckstein D. (2004). Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fennica 38: 167–178. http://dx.doi.org/10.14214/sf.426.
Schweingruber F.H., Bartholin T., Schar E., Briffa K.R. (1988). Radiodensitometric-dendroclimatological conifer chronologies from Lapland (Scandinavia) and the Alps (Switzerland). Boreas 17: 559–566. http://dx.doi.org/10.1111/j.1502-3885.1988.tb00569.x.
Skrøppa T. (2003). Technical guidelines for genetic conservation and use for Norway spruce Picea abies. EUFORGEN technical guidelines for genetic conservation and use.
Tegel W., Vanmoerkerke J., Büntgen U. (2010). Updating historical tree-ring records for climate reconstruction. Quaternary Science Reviews 29: 1957–1959. http://dx.doi.org/10.1016/j.quascirev.2010.05.018.
Vaganov E.A., Hughes M.K., Kirdyanov A.V., Schweingruber F.H., Silkin P.P. (1999). Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400: 149–151. http://dx.doi.org/10.1038/22087.
Total of 33 references

