Biomass production of coppiced grey alder and the effect of fertilization
Hytönen J., Saarsalmi A. (2015). Biomass production of coppiced grey alder and the effect of fertilization. Silva Fennica vol. 49 no. 1 article id 1260. https://doi.org/10.14214/sf.1260
Highlights
- Fertilisation (wood ash, N, PK) did not affect grey alder biomass production
- Leafless above-ground biomass of 17–20 year old stands was 52–57 Mg ha–1
- MAI increased with increase of rotation length to the end of the follow-up period of 17–20 years
- Coppicing increased stand density manifold.
Abstract
We studied biomass production of two naturally originated grey alder (Alnus incana (L.) Moench) stands having a mixture of birch and willow located in central Finland. One of the stands was growing on a peatland site (Muhos) and the other on a mineral soil site (Juuka). The stands were clear-cut and fertilization experiments were laid out with several treatments. At Muhos, the treatments included nitrogen fertilisation with different amounts of wood ash and an unfertilized control. At Juuka, the treatments included nitrogen fertilisation either with ash or with PK, and ash and PK treatments alone and an unfertilized control. The sprouts at Muhos were grown for 17 years and at Juuka for 20 years. At Juuka the stand was clear-cut second time at the age of 20 years and grown for 8 years. The stands were measured several times and foliar samples were taken twice during the study period. Clear-cutting increased stem number manifold. The stand density of new coppiced forests after the clear-cutting decreased from 67 000–89 000 stems ha–1 at the age of 3–6 years to 10 000–12 000 stems ha–1 at the age of 17–20 years. On neither site fertilization affected biomass production of alders during the study period. Leafless above-ground biomass was 52–57 Mg ha–1 after 17–20 years. Mean annual leafless above-ground biomass production (MAI) increased with increase of rotation time. At the age of 17–20 years the MAI was 2.8–3.0 Mg ha a–1. At Muhos, ash increased foliar P and Ca concentrations, but decreased those of Mn.
Keywords
biomass production;
coppicing;
grey alder;
fertilisation
Received 3 October 2014 Accepted 18 December 2014 Published 14 January 2015
Views 185143
Available at https://doi.org/10.14214/sf.1260 | Download PDF

1 Introduction
The use of bioenergy is increasing rapidly due to the need for reducing greenhouse gas emissions. Wood based fuels are playing a leading role in Finland in attempts to reach national and European Union goals for increasing the use of renewable energy. The National Climate and Energy Strategy indicate that annual production of forest chips in Finland is to be increased to 13.5 million m3 by the year 2020 (Ministry of Employment and the Economy 2010).
Woody biomass in mainly collected from existing forests as small sized trees from thinning stands and as slash and stumps from clear-cutting areas. Also growing of so called energy forests may become feasible and economically viable. Energy plantations based on fast growing deciduous tree species grown in dense stands and renewed by coppicing have been studied widely in various countries all over the world. Main emphasis in these studies has been in short-rotation willow plantations but also other deciduous tree species, such as alders and birches could be feasible with longer rotations.
Grey alder (Alnus incana (L.) Moench) is a pioneer tree species being fast growing at young age and able to regenerate from stump sprouts and root suckers (Heikinheimo 1917; Turunen 1953; Paukkonen et al. 1992). Grey alder is well adapted to growth conditions prevailing in temperate and boreal regions (Rytter 1996). In Finland grey alder dominated stands are mainly located on rich sites (Hakkila 1970) and their mean annual increment can be at the age of 5 years 5 m3 ha–1 (Miettinen 1932). In favorable conditions grey alder can reach height of 20 meters. The annual growth of grey alder seems to culminate at the age of 15–20 years (Miettinen 1932; Aosaar et al. 2012), but in some cases stands older than 20 years have still had high woody biomass production (Uri et al. 2014). Alder stands over 40 years of age often show signs of decay. Thus, if higher stemwood production than with Scots pine, Norway spruce or downy birch is desired the rotation length of grey alder stands has to be rather short (Miettinen 1932; Aosaar et al. 2012; Uri et al. 2014). The advantage of alder is also that it is not susceptible to damages by insects and mammals (vole, moose, hare) as e.g. birches, willows, aspen and poplar are.
Alders are considered biologically valuable tree species because of their ability to bind atmospheric nitrogen (e.g. Virtanen 1957; Mikola 1966; Aosaar et al. 2013; Uri et al. 2014). This is made possible by the symbiotic Frankia bacteria in alder root nodules. The estimates on the annual amounts of nitrogen bound by alders vary considerably e.g. by the age of trees, stand density and site types. Symbiotic nitrogen fixation in 5–30-year-old grey alder stands in Estonia, Norway and Sweden has been estimated to be 42–150 kg ha–1 a–1 (Johnsrud 1978; Rytter et al. 1990; Uri et al. 2011, 2014). Symbiotic nitrogen fixation may present 55–75% of the annual uptake of nitrogen by the stand (Rytter et al. 1990; Uri et al. 2011). In an alder stand the pH of the upper soil layer is often quite low, which is caused by acidification effects of nitrogen fixation, decomposition of litter and nitrification (Franklin et al. 1968; Tarrant and Trappe 1971; Borman and DeBell 1981).
Nutrients in deciduous trees are retranslocated from senescent leaves to other parts of the trees in autumn before the falling of leaves. The importance of internal nutrient cycling varies by tree species (Zimka and Stachurski 1976; Lennon et al. 1985). Generally 30% of the nitrogen need of deciduous tree species is covered by internal nutrient cycling (Cole and Rapp 1980). Compared to other tree species alder is quite wasteful in retranslocating nitrogen from senescent leaves (Viro 1955). Since alder can bind atmospheric nitrogen in its root nodules alders have not had selection pressure to develop processes to save nitrogen. The alder litter nitrogen content is generally 2–3 times as high as that of other European tree species (Mikola 1954, 1966; Viro 1955; Saarsalmi et al. 1985; Saarsalmi and Mälkönen 1989). In alder litter 50–80 kg ha–1 a–1 nitrogen is returned back to soil (Turner et al. 1976; Saarsalmi et al. 1985; Saarsalmi and Mälkönen 1989). Due to its high nitrogen and low lignin content the alder litter is easily decomposable (Mikola 1958) and thus alders have a soil ameliorating effect (Viro 1955; Virtanen 1957; Mikola 1958, 1966; Schalin 1966). Nitrogen is also released from alder roots to soil (Virtanen 1957; Aosaar et al. 2013; Uri et al. 2014).
Despite the benefits of alder, it has not interested forest growers since its use for other purposes than fuel wood has been modest. In Finland alder stands have been regenerated with economically more valuable tree species leading to a decrease in the share of alder dominated stand. In the 9th National Forest Inventory (1996–2000) the area of alder dominated stands in south Finland was 0.6% of the forest land area (Peltola 2001). In the inventories after that the share of alder stands have not been determined separately, but included into deciduous trees.
In Finland, the possibilities of growing grey alder for energy purposes has been studied in natural alder stands, alder plantations and sprout originated stands (Saarsalmi et al. 1985, 1991, 1992; Saarsalmi and Mälkönen 1989; Hytönen et al. 1995; Hytönen and Saarsalmi 2009). The biomass production of planted alder stands can be high (e.g. Aosaar et al. 2013). However, according to review of Aosaar et al. (2012) there are only few studies on the biomass production of naturally regenerated grey alder stands in Scandinavia and Baltic area. Because of good sprouting ability of grey alder the next alder generation can be established after clear-cutting from root suckers and stump sprouts. The good coppicing ability of grey alder was shown in a study in which stools which had been coppiced once before sprouted much better than those coppiced for the first time (Paukkonen et al. 1992). Thus repeated coppicing can improve the sprouting ability of grey alder.
In spite of atmospheric nitrogen fixation ability of alders a small amount of nitrogen is known to promote the growth of root nodules and to increase the efficiency of nitrogen fixation (Zavitkovski and Newton 1968), but upper limit for positive response is not known. On the other hand, the amount of root nodules and hence nitrogen fixation seems to decrease on sites rich in nitrogen (Quispel 1958; Huss-Danell 1980; Ingestad 1980; Palmgren et al. 1985). Thus the effect of nitrogen fertilization on the growth of alder can even be negative (Saarsalmi et al. 1992). Besides molybdenum, also phosphorus has a central role in nitrogen fixation due to the high phosphorus content of energy producing compound ATP (Sprent 1979). Phosphorus fertilization had not affected biomass production of alder plantations established on former agricultural plantations usually rich in phosphorus (Saarsalmi et al. 1985, 1992). On the other hand, on cutaway peatland areas phosphorus fertilization has increased alder biomass (Hytönen and Saarsalmi 2009). Also on forested mineral soil after whole-tree harvesting, phosphorus fertilization has increased biomass production of coppiced alder stand (Saarsalmi et al. 1991). Hence, it is obvious that also on forested mineral soils fertilization may be needed when biomass is harvested repeatedly.
The consumption of primary biomass for energy production is generating increasing quantities of wood ash. In Finland, the total amount of wood ash produced annually by the forest industry and heating plants is estimated to be 200 000–300 000 Mg. Wood ash contains all the major mineral nutrients essential for plant growth, except N. Maximum benefit from wood ash fertilization is obtained in stands on N-rich peatland sites where the main factors limiting tree growth are usually a lack of P and K (Moilanen et al. 2002, 2004). When growing alder either on cutaway peatlands or on forested mineral soils especially in the case the biomass is harvested repeatedly the need of phosphorus could be compensated by adding wood ash.
The aim of this study was to investigate the effect of fertilization on biomass production and nutrition of coppiced grey alder stands on both peat soil and mineral soil. We hypothesized that wood ash would increase the growth of alders, and that the effect of additional nitrogen would be small.
2 Materials and methods
Two experiments were established in natural dense alder dominated stands: one was located on a peatland site at Muhos (64°52´N, 26°4´E, 75 m a.s.l.), northern Ostrobothnia and the other on a mineral soil site at Juuka (63°3´N, 29°40´E, 130 m a.s.l.), North Carelia (Fig. 1).
Fig. 1. Location of the experiments.
2.1 Muhos experiment
Before establishment of the experiment the 3–6 m high dense mixed alder-birch stand at Muhos had 22 670 stems ha–1 (alder 24%, downy birch 37%, willow 39%) and was estimated to be 20-years old. The stand was growing on tall-sedge hardwood-spruce fen drained in 1967 having a shallow peat layer of 20–30 cm (mean 18 cm). Altogether 12 sample plots (size 195–371 m2) were established in the stand. On the plot lines shallow ditches were dug with a shovel to eliminate roots penetrating other plots. All the trees on the sample plots were clear-cut at the end of April 1979 to about 30 cm long stumps. The harvested trees were removed from the plots.
Fertilization experiment with six treatments replicated twice consisting of an unfertilized control and ash doses from 0.5 Mg ha–1 to 10 Mg ha–1 was set up in the beginning of October 1979 (Table 1). The ash was dry bark ash from Oulu Oy. Together with all ash doses 96 kg N ha–1 as urea was applied. The treatments with 0.5 and 1.0 Mg ha–1 of ash were re-fertilized at the end of May 1986 with urea and PK-fertilizer in order to study the response of alders to very high amount of N fertilizer.
| Table 1. Fertilization treatments. Amounts of main nutrients in the treatments. | ||||||
| Code | N | P | K | Ca | Mg | |
| kg ha–1 | ||||||
| 1. | 0 | 0 | 0 | 0 | 0 | 0 |
| 2.1) | A0.5+N | 93 | 6 | 11 | 160 | 19 |
| 3.1) | A1.0+N | 93 | 13 | 21 | 319 | 38 |
| 4. | A2.5+N | 93 | 31 | 53 | 798 | 94 |
| 5. | A5.0+N | 93 | 63 | 105 | 1595 | 88 |
| 6. | A10.0+N | 93 | 125 | 210 | 3190 | 375 |
| A = Ash (P 13 g kg–1, K 21 g kg–1, Ca 319 g kg–1, Mg 37.5 g kg–1, B 226 mg kg–1, Cu 212 mg kg–1, Zn 278 mg kg–1, Mn 21 g kg–1). A0.5 = 0.5 Mg ha–1, A1.0 = 1 Mg ha–1, A2.5 = 2.5 Mg ha–1, A5.0 = 5 Mg ha–1, A 10.0 = 10 Mg ha–1. N = Urea 200 kg ha–1 (N 46.3%) 1) Treatments 2 and 3 re-fertilized in 1986 with urea (N 185 kg ha–1) and PK (P 36 kg ha–1, K 68 kg ha–1). | ||||||
The trees were measured when they were 3, 8 and 17 years-old from circular sub-plots (50 m2 or 100 m2 in Spring 1982 and 38–100 m2 in Spring 1996 and from 5 sub-plots, each 5 m2 in Autumn 1986) on each sample plot. The height (cm) and diameter (mm, basal diameter d0.1, and in 1996 also breast height diameter (d1.3) of trees d1.3 > 5 cm) of each tree was measured. No trees damaged by animals (voles, hares, moose) were observed. Peat depth was measured from 5 locations on each plot.
Altogether 60 biomass sample trees (20 alders, 20 downy birches, 20 willows), selected according to the stand diameter distribution, were felled in spring 1982. After felling, the tree basal diameter (d0.1) and height were measured. The branches and stems of the alder and birch sample trees were weighed separately. Due to their bushy growth form willow stems and branches were not separated. The samples were dried to a constant weight in laboratory at 105 °C. The above-ground woody biomass (stem and branch dry mass) of the trees was calculated using allometric biomass equations (Table 2).
| Table 2. Leafless above-ground biomass equations for three-year old alders, downy birches and willows. Equations have the form Y = aXb, and have been corrected after logarithmic transformation with se2/2. Y = dry mass (g), X = d0.1, d0.1 = basal diameter (mm), a and b = constants, R2 = coefficient of determination, N = number of sample trees. | ||||
| Species | a | b | R2 % | N |
| Alnus incana | 0.099 | 2.364 | 98.7 | 20 |
| Betula pubescens | 0.225 | 2.186 | 96.7 | 20 |
| Salix spp. | 0.089 | 2.598 | 97.8 | 20 |
The biomass for 8-year old trees was calculated with equations presented in Table 2 when the trees were shorter than 1.3 m and breast height diameter was not measured. For alders taller than 1.3 m biomass equations presented by Johansson (2000) and for downy birch and willow biomass by equations presented by Hytönen and Kaunisto (1999) were used (at Muhos and at Juuka). The equations of Johansson (1999) were considered to be suitable for this study since they are based on sample trees from 15 dense stands having similar age variation than in our study. Also the birch equations used come from dense stands with similar age structure.
2.2 Juuka experiment
The dense alder dominated stand at Juuka was 4–10 m in height and had some spruce and pine trees as mixture. Alders constituted 98.2% of the biomass and birches 1.5%. The area has been used for cattle grazing and the mineral soil forest site type was fertile Myrtillys type – Oxalis Myrtillys type according to the Finnish site type classification (Cajander 1926). Altogether 15 sample plots (size 225 m2) were established in the stand. The stand was clear-cut in autumn 1980 and the harvested trees were removed from the plots. Oldest trees were 34-years old. The fertilization experiment was established at the end of October 1980. The treatments included an unfertilized control, PK, NPK, and wood ash fertilization either alone or with N (Table 3). The five treatments were repeated on randomized blocks three times. The wood ash was from Vihanti power plant.
| Table 3. Fertilization treatments at Juuka. The amount of main nutrients in each treatment. | ||||||
| Code | N | P | K | Ca | Mg | |
| kg ha–1 | ||||||
| 1. | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. | PK | 0 | 45 | 85 | 118 | 2 |
| 3. | NPK | 93 | 45 | 85 | 118 | 2 |
| 4. | Ash3 | 0 | 74 | 242 | 663 | 108 |
| 5. | Ash3 + N | 93 | 74 | 242 | 663 | 108 |
| A = Ash 3000 kg ha–1 (P 25 g kg–1, K 81 g kg–1, Ca 221 g kg–1, Mg 36 g kg–1, B 356 mg kg–1, Cu 510 mg kg–1, Zn 2627 mg kg–1, Mn 25 g kg–1, Fe 28 g kg–1). N = Urea 200 kg ha–1 (N 46.3%) PK = PK fertilizer for peatlands 500 kg ha–1 (N 0%, P 9%, K 17%, Ca 23%, Mg 0.3%, B 0.02%) | ||||||
The trees with d1.3 > 0.5 cm were measured before clear-cutting in the beginning of September 1980 from circular sub-plots (100 m2) on each sample plot. After clear-cutting the sprouts were measured at the age of 6 years (autumn 1986) and 20 years (autumn 2000). The height (cm) and diameter (d0.1, d1.3) of the 6-year-old sprouts were measured from 5 circular sub-plots (5 m2) on each sample plot. The 20-year-old stands were measured using larger (100 m2) circular sub-plots on each sample plot. The diameter (d1.3) of all trees with d1.3 > 1.5 cm was measured, and damages of the alders were recorded. No trees damaged by animals (voles, hares, moose) were found. Height of every 7th tree was measured (altogether 439 trees). The trees were clear cut after the measurements and let to sprout. After second clear-cutting the sprouts were measured at the age of 9 years (Autumn 2009). The diameters (d1.3) of all sprouts higher than 1.3 m were measured from circular sub-plots (50 m2) on each sample plot. Height of every 7th tree was measured (altogether 530 sample trees).
2.3 Foliar samples
In late august 1984 and 1986 foliar samples were taken from Muhos experiment from 10 grey alders from each plot. From Juuka experiment foliar samples were taken in mid-august 1990 and in beginning of September 2000 from six trees from each plot. In both experiments leaves from sun-exposed upper whorls from randomly selected trees were sampled. The foliar nitrogen, phosphorus, potassium, calcium, magnesium, manganese, zinc, copper and boron concentrations were analyzed with standard methods (Halonen et al. 1983) at the Muhos and Kannus laboratories of the Finnish Forest Research Institute.
2.4 Statistical analysis
Dominant diameter was calculated as the mean diameter of 100 largest trees ha–1 and dominant height as their mean height. Two-way analysis of variance was used to study the effects of treatments on the measured variables (treatment, block). The effect of fertilization on the foliar nutrient concentrations was studied with repeated measures analysis of variance. When stand characteristics were analyzed the stem number in the mother stand before clear-cutting was used as covariate. Tukey’s test was used in post hoc pairwise multiple comparisons between treatments except in the case of foliar nutrients Dunnett’s test was used to find out treatments differing from control. All analyses were computed using the IBMM SPSS Statistics 20 package.
3 Results
3.1 Stand density
Fertilization treatments did not affect the stand density at Muhos (p3a = 0.529, p8a = 0.840, p17a = 0.104) or at Juuka (p6a = 0.051, p20a = 0.392, p9a = 0.544). Thus mean stand densities of all plots are used to describe the development of stem numbers with age (Fig. 2).
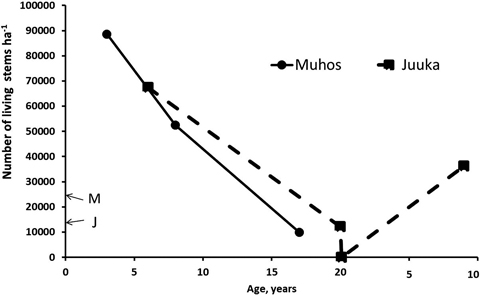
Fig. 2. Total number of living stems after clear-cutting at Muhos and Juuka. At age 0 letter M (Muhos) and J (Juuka) indicate the density of mother stands before clear-cutting. As fertilisation treatments did not affect stand density, the results are presented as means of all plots.
Three years after clear-cutting total number of all living sprouts at Muhos was 88 640 stems ha–1 (Fig. 2). The number of alder sprouts had increased fivefold, to 28 500 stems ha–1, and that of birch and willow 4.6 and 2.4 fold, respectively. At the age of 8 years the stand density had decreased to 52 470 stems ha–1. The number of alder stems had decreased by 33% to 19 070 stems ha–1, and those of birch and willow stems by 46% and 47%, respectively. When the final measurement was done at the age of 17 years the number of stems was 9960 ha–1. The number of alder stems had decreased by 75% from the number of alder stems three years after clear-felling to 7120 stems ha–1. The number of birch stems had decreased even more by 93% and that of willow stems by 99% leaving only 89 willow stems ha–1.
In the Juuka mother stand before clear-cutting there were 14 020 stems ha–1 (Fig. 2) being mostly alders (93%) with some birches and other tree species. After clear-cutting at the age of 6 years the stand density was 67 360 stems ha–1. The number of alder sprouts had increased 3.7 fold to 48 960 stems ha–1. The number of birches had increased from 600 to 13 010 stems ha–1 and that of willows from 0to 450 stems ha–1. At the age of 20 years there were 12 080 stems ha–1. Alders dominated (74%) with 8990 stems ha–1. The share of downy birches had decreased to 2510 stems ha–1 (21%). There were no willows left at the stand at the age of 20 years. The stand was clear-cut again and left to sprout. When re-measured at the age of 9 years, the stand density was 36 170 stems ha–1. Alders dominated the stand (75%) with mixture of birch (13%). The stands at Muhos self-thinned faster than the stand at Juuka.
3.2 Height and diameter
Fertilization treatments did not affect the mean height, mean diameter, dominant height and dominant diameter significantly at Muhos (mean h: p3a = 0.182, p8a = 0.076, p17a = 0.151; mean d0.1: p3a = 0.384, p8a = 0.125, p17a = 0.080 ). At Juuka fertilization had a significant effect of the heights measured after 6 growing seasons (mean h: p6a = 0.044*, p20a = 0.155, p9a = 0.820; mean d1.3: p6a = 0.071, p20a = 0.145, p9a = 0.552). Trees fertilized with Ash+N were after 6 years significantly taller and had higher diameter than unfertilized trees. Other fertilization treatments did not differ significantly from each other. The mean height of Ash+N fertilized alders and birches was 0.9 m and 1.2 m higher than those of unfertilized alders and birches, respectively. However, there were no significant differences in the following measurements between the fertilization treatments.
The development of height and diameter (mean of all plots) of alders was better on the peatland site at Muhos than on the mineral soil site at Juuka (Fig. 3). Dominant height and diameter were much higher than mean figures due to high number of small stems in the stands. In the final measurements at the age of 17–20 years mean height of alders was 6.5–6.6 m and their dominant height 60–70% greater (10–11 m). At the age of 17–20 years the mean diameter was 4–5 cm and their dominant diameter was 12 cm. Difference between mean diameter and dominant diameter was bigger than difference between mean height and dominant height.
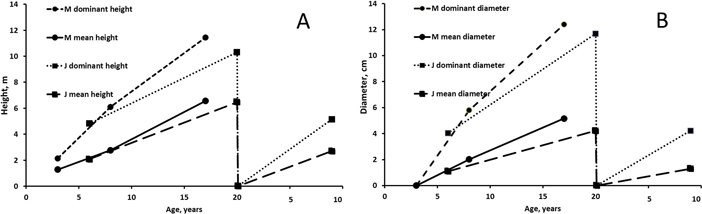
Fig. 3. Mean and dominant height (A) and mean and dominant breast height diameter (B) of alders at Muhos (M) and Juuka (J). As fertilisation did not affect tree characteristics except in one case (at Juuka after 6 years), the results are presented as means of all plots. View larger in new window/tab.
3.3 Biomass production
Fertilization treatments did not affect the leafless above-biomass production of the stands at Muhos (p3a = 0.125, p8a = 0.083, p17a = 0.117) or at Juuka (p6a = 0.882, p20a = 0.904, p9a = 0.458). Since fertilization did not affect biomass production mean of all treatments was calculated (Fig. 4). At Muhos the leafless above-ground biomass of the stands reached at the age of 17 years 52 Mg ha–1 (Fig. 4). The share of alder was 73%. At Juuka the biomass of c. 37-year-old mother stand was 35 Mg ha–1 and after coppicing, at the age of 20 years, the biomass reached 57 Mg ha–1 The mother stand was almost pure alder forest (98% alder biomass), but after coppicing the share of alder decreased and was at the age of 20-years 57%. In both experiments mean annual biomass production (MAI) increased with increase of age. The MAI of the stands at Muhos and Juuka was 1.6–1.9 Mg ha–1 at the age of 6–8 years and 2.8–3.0 Mg ha–1 at the age of 17–20 years. At the age of 17–20 years 4.1- 5.1% of the biomass consisted of dead trees.
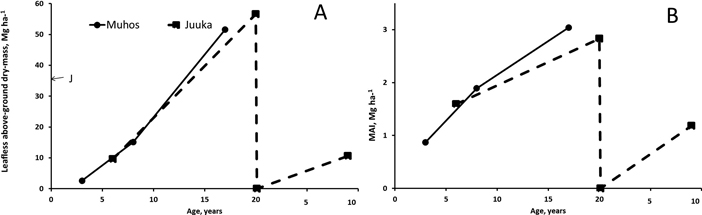
Fig. 4. Leafless above-ground biomass production (A) and mean annual leafless biomass production (B) at Muhos and at Juuka. J at age 0 marks the biomass of the mother stand at Juuka before clear-cutting. As fertilisation did not affect the biomass production, the results are presented as means of all plots. View larger in new window/tab.
The biomass at the age of 17 (Muhos) or 20 (Juuka) years did not correlate with the stand density of all trees (rMuhos = 0.449, p = 0.095, rJuuka = 0.320, p = 0.311). However, when stand density of trees with d1.3 > 5 cm was calculated the stand density correlated significantly with leafless above-ground biomass of the stands both at Muhos and at Juuka (Fig. 5).
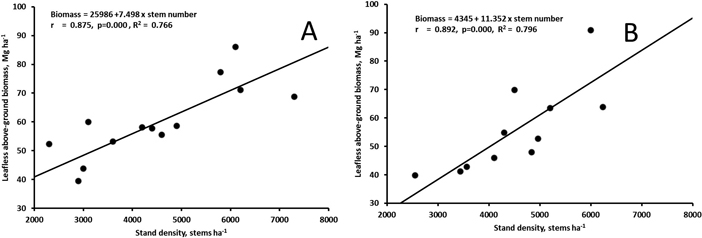
Fig. 5. Correlation between leafless above-ground biomass and the number of stems with d1.3 > 5 cm in the 17-year old stand at Muhos (A) and in the 20-year old stand at Juuka (B). View larger in new window/tab.
3.4 Foliar nutrient concentrations
Fertilization treatments did not affect foliar N, K, Fe, Zn and B concentrations at Muhos (Table 4). However, fertilization increased foliar P, Ca and Mg concentrations the more ash had been used but decreased foliar Mn concentrations. At Juuka, however, foliar Mn concentrations were significantly higher in the Ash3 treatment than in the control treatment (Table 5). Significant differences between treatments were also found in overall tests in foliar Cu concentrations, but these were not seen between any particular treatment.
| Table 4. Foliar nutrient concentrations of alder sprouts at Muhos. The results are means for the leaves sampled in autumn 1984 and 1986, i.e., 5 and 7 years after the first fertilization. The treatments differing significantly from the unfertilized control (0) according to Dunnett’s test are marked with asterisks. Significance of F-values marked as * p ≤ 0.5, ** p < 0.01, *** p ≤ 0.001). | |||||||
| Nutrient | Fertilization treatments 1) | ||||||
| 0 | Ash0.5+N | Ash1+N | Ash2.5+N | Ash 5+N | Ash10+N | F-value | |
| N, mg g–1 | 29.7 | 28.4 | 31.2 | 29.9 | 30.7 | 30.4 | 0.656 |
| P, mg g–1 | 1.70 | 1.91 | 2.04 | 2.65* | 2.46* | 2.56* | 6.579** |
| K, mg g–1 | 8.45 | 7.82 | 9.05 | 8.41 | 8.06 | 9.97 | 1.453 |
| Ca, mg g–1 | 7.84 | 7.69 | 7.56 | 8.25 | 10.10* | 10.56* | 7.167** |
| Mg, mg g–1 | 2.56 | 2.65 | 2.31 | 2,93 | 2.84 | 2,91 | 3.515* |
| Mn, ppm | 812 | 806 | 652 | 395* | 338* | 289* | 15.211*** |
| Fe, mg kg–1 | 67.2 | 67.5 | 71.8 | 58.4 | 69.5 | 69.8 | 1.130 |
| Zn, mg kg–1 | 79.9 | 85.1 | 89.4 | 86.0 | 74.9 | 69.2 | 0.487 |
| Cu, mg kg–1 | 9.0 | 7.7 | 12.8 | 7.8 | 7.5 | 6.7 | 4.699* |
| B, mg kg–1 | 25.2 | 26.0 | 26.1 | 30.9 | 33.7 | 36.7 | 4.200 |
| 1) Treatments Ash 0.5 + N and Ash 1+N were re-fertilized in May 1986 with NPK. | |||||||
| Table 5. Foliar nutrient concentrations of alder sprouts at Juuka. The results are means for leaves sampled in autumn 1990 and 2000, i.e. 10 and 20 years after fertilization. The treatments differing significantly from the unfertilized control (0) according to Dunnett’s test are marked with asterisks. Significance of F-values marked as * p ≤ 0.5, ** p < 0.01, *** p ≤ 0.001). | ||||||
| Nutrient | Fertilization treatment | |||||
| 0 | PK | NPK | Ash3 | Ash3+N | F-value | |
| N, mg g–1 | 33.6 | 33.5 | 33.0 | 32.6 | 33.0 | 0.454 |
| P, mg g–1 | 1.79 | 1.92 | 2.01 | 1.67 | 1.81 | 2.208 |
| K, mg g–1 | 8.67 | 8.07 | 9.08 | 8.10 | 8.10 | 0.885 |
| Ca, mg g–1 | 6.63 | 7.15 | 5.52 | 7.08 | 6.84 | 1.082 |
| Mg, mg g–1 | 2.00 | 2.16 | 1.94 | 1.80 | 1.89 | 1.036 |
| Mn, ppm | 263 | 434 | 197 | 548* | 428 | 4.531* |
| Fe, mg kg–1 | 53.3 | 54.2 | 55.8 | 51.8 | 53.4 | 0.368 |
| Zn, mg kg–1 | 27.4 | 28.2 | 23.9 | 34.6 | 30.6 | 1.466 |
| Cu, mg kg–1 | 11.1 | 8.6 | 9.6 | 9.8 | 9.3 | 3.790* |
| B, mg kg–1 | 15.9 | 8.9 | 15.7 | 12.5 | 15.2 | 0.841 |
4 Discussion
In bioenergy plantations high stand density is often seen as a prerequisite for attaining high yields in short rotations. Naturally regenerated young stands of grey alder can have high stand densities (15 000 to 50 000 stems ha–1 at 5–10 years) (Björklund and Ferm 1982; Aosaar et al. 2012). Good sprouting ability is an advantage for establishment of second rotation with minimum costs. Grey alder seems to be suitable species in these respects. Our study demonstrates that following clear-cutting, the stem number had increased manifold compared (67 000–89 000 stems ha–1 at the age of 3 to 6 years) to the density of the mother stands indicating good sprouting ability. With increasing age self-thinning proceeds and stand density will decrease. At the age of 20 years most seed originated grey alder stand have less than 5000 stems ha–1 (Aosaar et al. 2012) i.e. clearly less than in our stands at the same age (10 000–12 000 stems ha–1). Our study indicates that after clear-cutting alder stands can have much higher density than first generation seed originated stands and self-thinning proceeds slower than in seed originated stands.
Nitrogen fertilization combined with ash did not increase the biomass production of grey alder. This is in agreement with previous studies carried out in planted dense alder stands (Saarsalmi et al. 1985; Rytter et al. 1989). In a study by Saarsalmi et al. (1992) nitrogen fertilization even decreased biomass production. This was due to increased mortality caused by nitrogen fertilization observed also by Kaunisto and Viinamäki (1991).
Phosphorus fertilization has increased biomass production of grey alder, e.g. in a coppiced stand 5- and 8 years after whole-tree harvest on a mineral soil site (Saarsalmi et al. 1991). On forested mineral soils the N/P ratio decreases with increasing soil fertility (Tamminen and Mälkönen 2003). This might explain the positive response of P fertilization on biomass production of the coppiced alder stand, rich in N, detected by Saarsalmi et al. (1991). Also, on a phosphorus poor cutaway peat PK fertilization significantly increased the leafless above-ground biomass production of grey alder (Hytönen and Saarsalmi 2009). However, in our study P added in ash, or in PK fertilizer did not affect alder biomass production either on the peatland or the mineral soil site.
The mean annual leafless biomass production (MAI) of the studied coppiced alder stands increased with increasing age. At the age of 17–20 years the MAI was at the same level as in most previous studies (Fig. 6). Although Johansson (2000) found very high biomass production in some alder stands, production in most of the stands were similar as measured in our study. It seems that alders are not suitable for growing on short rotations, i.e. less than 10 years. An optimal rotation length of dense planted grey alder stands has been estimated to be 15–20 years (Uri et al. 2011; Aosaar et al. 2012, 2013). Biomass production of planted dense grey alder stands reported in previous studies (e.g. Aosaar et al. 2012, 2013; Uri et al. 2002, 2003) has been considerably higher than measured in natural alder stands of our study.
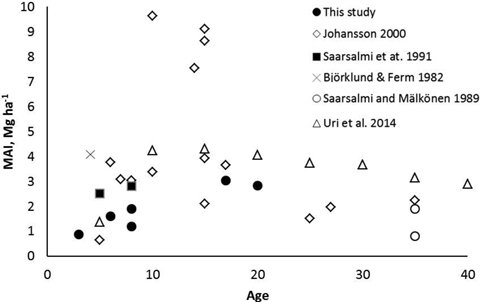
Fig. 6. Mean annual leafless above-ground biomass production (MAI) of naturally originated grey alder stands in Scandinavian and Baltic studies and in this study.
Compared to the results obtained in this study for alder, in dense unthinned downy birch stands similar or slightly higher leafless above-ground biomass production figures have been reported in Finland (2.5–5.3 Mg ha–1 a–1, Björklund and Ferm 1982; Ferm 1990; Hytönen and Aro 2012; Niemistö 2013), in Ireland (3.1. to 5.8 Mg ha–1 a–1, Renou-Wilson et al. 2010) and in Sweden (0.5 to 4.4 Mg ha–1 a–1, Johansson 1999).
In this study the total stand density did not correlate with biomass measured in the sample plots. However, the number of stems with d1.3 > 5 cm correlated well with biomass of the plots in 17–20-year-old stands. This indicates that even in older stands there may still be a lot of small sized stems which contribute only little to the biomass production. Biomass production largely depends on the number of stems forming the upper canopy. Thus low number of stems > 5 cm may have restricted production in the study. In the densest plots at the age of 17–20 years the MAI was at Muhos over 5 Mg ha–1 and at Juuka over 4 Mg ha–1 showing the high biomass production potential of grey alder. However, this shows also that one should be cautious when generalizing biomass production estimates derived from small plots to larger areas.
Except for high Mn concentration at Juuka, foliar nutrient concentrations did not differ from those reported in previous studies for grey alder (Viro 1955; Saarsalmi and Mälkönen 1989; Saarsalmi et al. 1985, 1991, 1992; Hytönen et al. 1995). Neither any great difference in foliar nutrient concentrations between the studied sites could be found, except concentrations of Mn, Zn and B which were higher on the peatland site at Muhos than on the mineral soil site at Juuka. In fact, foliar Mn concentrations at Muhos were clearly higher compared to those reported for alder leaves in previous studies (Saarsalmi et al. 1985, 1991, 1992; Hytönen et al. 1995). Similar wood ash induced decreases in foliar Mn concentrations detected at Muhos after the ash doses 2.5 to 10 Mg ha–1, has also been reported in Scots pine needles on mineral soil sites (Saarsalmi et al. 2004, 2005; Moilanen et al. 2013). Unclear remain, however, why wood ash increased foliar Mn concentrations at Juuka.
5 Conclusions
Alder’s capability of fixing atmospheric nitrogen, the good coppicing ability and fast growth are characteristics that affect directly the economy of biomass production. Our study confirmed that nitrogen fertilization does not increase the biomass production of grey alder. Even though nitrogen does not increase biomass production, the use of phosphorus given in wood ash, especially after repeated harvesting on different sites, needs further study. The advantage of alder is also that it is not susceptible to damages by insects and mammals (vole, moose, hare) as e.g. birches, willows, aspen and poplar are. The biomass production of grey alder was comparable or even higher than that of birches. Alder stands can be coppiced several times, in our study its good coppicing ability was shown by the good growth of third generation alder stand. However, grey alder is not suitable for very short rotations.
Acknowledgements
We want to thank Jorma Issakainen and Eero Saari for carrying out the experiment measurements. Seppo Vihanta also took part in the calculation of the results.
References
Aosaar J., Varik M., Lõhmus K., Ostonen I., Becker H., Uri V. (2013). Long-term study of above- and below-ground biomass production in relation to nitrogen and carbon accumulation dynamics in a grey alder (Alnus incana (L.) Moench) plantation on former agricultural land. European Journal of Forest Research 132(5): 737–749. http://dx.doi.org/10.1007/s10342-013-0706-1.
Aosaar J., Varik M., Uri V. (2012). Biomass production potential of grey alder (Alnus incana (L.) Moench) in Scandinavia and Eastern Europe: a review. Biomass & Bioenergy 45: 11–26. http://dx.doi.org/10.1016/j.biombioe.2012.05.013.
Björklund T., Ferm A. (1982). Pienikokoisen koivun ja harmaalepän biomassa ja tekniset ominaisuudet. Summary: Biomass and technical properties of small-sized birch and grey alder. Folia Forestalia 500. 37 p.
Bormann B.T., De Bell D.S. (1981). Nitrogen content and other soil properties related to age of red alder stands. Soil Science Society of American Journal 45: 428–432. http://dx.doi.org/10.2136/sssaj1981.03615995004500020038x.
Cajander A.K. (1926). The theory of forest types. Acta Forestalia Fennica 29(3). 108 p.
Cole D.W., Rapp M. (1980). Elemental cycling in forest ecosystems. In: Reichle D.E. (ed.). Dynamic properties of forest ecosystems. International Biological Programme 23. Cambridge University Press, Cambridge. p. 341–409.
Ferm A. (1990). Coppicing, aboveground woody biomass production and nutritional aspects of birch with specific reference to Betula pubescens. Metsäntutkimuslaitoksen tiedonantoja – Finnish Forest Research Institute, Research Reports 348. 35 p.
Franklin J.F., Dyrness C.T., Moore D.G., Tarrant R.F. (1968). Chemical soil properties under coastal Oregon stands of alder and conifers. In: Trappe J.M., Franklin J.F., Hansen G.M. (eds.). Biology of alder. USDA Forest Service, PNW Forest and Range Experimental Station, Portland, OR. p. 157–172.
Hakkila P (1970). Basic density, bark percentage and dry matter content of grey alder (Alnus incana). Communicationes Instituti Forestalis Fenniae 71(5). 33 p.
Halonen O., Tulkki H., Derome J. (1983). Nutrient analysis methods. Metsäntutkimuslaitoksen tiedonantoja – Finnish Forest Research Institute, Research Reports 121. 28 p.
Heikinheimo O. (1917). Metsien uudistuminen vesojen avulla. I. Suomen Metsänhoitoyhdistys Tapion julkaisema Aikakauskirja 10(2): 33–38.
Huss-Danell K. (1980). Nitrogen fixation and biomass production in clones of Alnus incana. New Phytologist 85(4): 503–511. http://dx.doi.org/10.1111/j.1469-8137.1980.tb00765.x.
Hytönen J., Aro L. (2012). Biomass and nutrition of naturally regenerated and coppiced birch on cutaway peatland during 37 years. Silva Fennica 46(3): 377–394. http://dx.doi.org/10.14214/sf.48.
Hytönen J., Kaunisto S. (1999). Effects of fertilization on the biomass production of coppiced mixed birch and willow stands on a cut-away peatland. Biomass and Bioenergy 17(6): 455–469. http://dx.doi.org/10.1016/S0961-9534(99)00061-6.
Hytönen J., Saarsalmi A. (2009). Long-term biomass production and nutrient uptake of birch, alder and willow plantations on cut-away peatland. Biomass & Bioenergy 33(9): 1197–1211. http://dx.doi.org/10.1016/j.biombioe.2009.05.014.
Hytönen J., Saarsalmi A., Rossi P. (1995). Biomass production and nutrient uptake of short-rotation plantations. Silva Fennica 29(2): 117–139. http://doi.org/10.14214/sf.a9202.
Ingestad T. (1980). Growth, nutrition and nitrogen fixation in grey alder at varied rate of nitrogen addition. Physiologia Plantarum 50: 353–364. http://dx.doi.org/10.1111/j.1399-3054.1980.tb04113.x.
Johansson T. (1999). Biomass equations for determining fractions of pendula and pubescent birches growing on abandoned farmland and some practical implications. Biomass & Bioenergy 16(3): 223–238. http://dx.doi.org/10.1016/S0961-9534(98)00075-0.
Johansson T. (2000). Biomass equations for determining fractions of common and grey alders growing on abandoned farmland and some practical implications. Biomass & Bioenergy 18(2): 147–159. http://dx.doi.org/10.1016/S0961-9534(99)00078-1.
Johnsrud S.C. (1978). Nitrogen fixation by root nodules of Alnus incana in a Norwegian forest ecosystem. Oikos 30(3): 475–479.
Kaunisto S., Viinamäki T. (1991). Lannoituksen ja leppäsekoituksen vaikutus mäntytaimikon kehitykseen ja suonpohjaturpeen ominaisuuksiin Aitonevalla. Summary: Effect of fertilization and alder (Alnus incana) mixture on the development of young Scots pine (Pinus sylvestris) trees and the peat properties in a peat cutover area at Aitoneva, southern Finland. Suo 42(1): 1–12.
Lennon J.M., Aber J.D., Melillo J.M. (1985). Primary production and nitrogen allocation of field-grown sugar maples in relation to nitrogen availability. Biogeochemistry 1(2): 135–154.
Miettinen L. (1932). Tutkimuksia harmaalepikoiden kasvusta. Referat: Untersuchungen über den Zuwachs der Weisserlenbestände. Communicationes Instituti Forestalis Fenniae 18(1). 86 p.
Mikola P. (1954). Kokeellisia tutkimuksia metsäkarikkeiden hajaantumisnopeudesta. Summary: Experiments on the rate of decomposition of forest litter. Communicationes Instituti Forestalis Fenniae 43(1). 50 p.
Mikola P. (1958). Liberation of nitrogen from alder leaf litter. Acta Forestalia Fennica 67 (1). 10 p.
Mikola P. (1966). The value of alder in adding nitrogen in forest soil. Final report of research conducted under grant authorized by U.P. Public Law 480. Library of Forestry, University of Helsinki, Finland. 95 p.
Ministry of Employment and the Economy (2010). Suomen kansallinen toimintasuunnitelma uusiutuvista lähteistä peräisin olevan energian edistämisestä direktiivin 2009/28/EY mukaisesti. [National action plan of Finland on the promotion of the use of energy from renewable sources following Directive 2009/28/EY]. 10 p.
Moilanen M., Silfverberg K., Hokkanen T.J. (2002). Effects of wood-ash on the tree growth, vegetation and substrate quality of a drained mire: a case study. Forest Ecology and Management 171(3): 309–320. http://dx.doi.org/10.1016/S0378-1127(01)00789-7.
Moilanen M., Silfverberg K., Hökkä H., Issakainen J. (2004). Comparing effects of wood ash and commercial PK fertiliser on the nutrient status and stand growth of Scots pine on drained mires. Baltic Forestry 10(2): 2–10.
Moilanen M., Saarsalmi A., Kukkola M., Issakainen J. (2013). Effects of stabilized wood ash on nutrient status and growth of Scots pine – comparison between uplands and peatlands. Forest Ecology and Management 295: 136–144. http://dx.doi.org/10.1016/j.foreco.2013.01.021.
Niemistö P. (2013). Effect of growing density on biomass and stem volume growth of downy birch stands on peatland in Western and Northern Finland. Silva Fennica 47(4): 1–24. http://dx.doi.org/10.14214/sf.1002.
Palmgren K., Saarsalmi A., Weber A. (1985). Nitrogen fixation and biomass production in some alder clones. A greenhouse experiment. Silva Fennica 19(4): 407–420. http://dx.doi.org/10.14214/sf.a15433.
Paukkonen K., Kauppi A., Ferm A. (1992). Root and stump sprouts as structural faculties for reinvigoration in Alnus incana (L.) Moench. Flora 187(5–6): 353–367.
Peltola A. (ed.). (2001). Finnish Statistical Yearbook in Forestry 2001. 372 p.
Quispel A. (1958). Symbiotic nitrogen fixation in non-leguminous plants IV. The influence of some environmental conditions on different phases of the nodulation process in Alnus glutinosa. Acta Botanica Neerlandica 7: 191–204.
Renou-Wilson F., Pöllänen M., Byrne K., Wilson D., Farrell E.P. (2010). The potential of birch afforestation as an after-use option for industrial cutaway peatlands. Suo – Mires and Peat 61(3–4): 59–76.
Rytter L. (1996). Grey alder in forestry. A review. Norwegian Journal of Agricultural Sciences. Supplement 24: 65–84.
Rytter L., Slapokas T., Granhall U. (1989). Woody biomass and litter production of fertilized grey alder plantations on a lowhumidified peat bog. Forest Ecology and Management 28(3–4): 161–176. http://dx.doi.org/10.1016/0378-1127(89)90001-7.
Rytter L., Arveby A.S., Granhall U. (1990). Dinitrogen fixation in relation to nitrogen fertilization of grey alder plantations on a peat bog. Biology and Fertility of Soils 10(4): 233–240.
Saarsalmi A., Mälkönen E. (1989). Harmaalepikon biomassan tuotos ja ravinteiden käyttö. Summary: Biomass production and nutrient removal in whole tree harvesting of birch biomass. Folia Forestalia 728. 16 p.
Saarsalmi A., Palmgren K., Levula T. (1985). Leppäviljelmän biomassan tuotos sekä ravinteiden ja veden käyttö. Summary: Biomass production and nutrient and water consumption in an Alnus incana plantation. Folia Forestalia 628. 24 p.
Saarsalmi A., Palmgren K., Levula T. (1991). Harmaalepän vesojen biomassan tuotos ja ravinteiden käyttö. Summary: Biomass production and nutrient consumption of the sprouts of Alnus incana. Folia Forestalia 768. 25 p.
Saarsalmi A., Palmgren K., Levula T. (1992). Harmaalepän ja rauduskoivun biomassan tuotos ja ravinteiden käyttö energiapuuviljelmällä. Summary: Biomass production and nutrient consumption of Alnus incana and Betula pubescens in energy forestry. Folia Forestalia 797. 29 p.
Saarsalmi A., Mälkönen E., Kukkola M. (2004). Effect of wood ash fertilization on soil chemical properties and stand nutrient status and growth of some coniferous stands in Finland. Scandinavian Journal of Forest Research 19(3): 217–233. http://dx.doi.org/10.1080/02827580410024124.
Saarsalmi A., Derome J., Levula T. (2005). Effect of wood ash fertilization on stand growth, soil, water and needle chemistry, and berry yields of lingonberry (Vaccinium vitis-idaea L.) in a Scots pine stand in Finland. In: Mandre M. (ed.). Utilisation of industrial wastes in forestry. Metsanduslikud Uurimused/Forestry Studies 42: 13–33.
Schalin I. (1966). Harmaalepän merkityksestä käytännön metsätaloudessa. Summary: Alnus incana (L.) Moench in forestry practice. Metsätaloudellinen Aikakauslehti 83(9): 362–366.
Sprent J.I. (1979). The biology of nitrogen fixing organisms. McGraw-Hill Company, London. 196 p.
Tamminen P., Mälkönen E. (2003). Metsämaiden viljavuus. In: Mälkönen E. (ed.). Metsämaa ja sen hoito. Metsäntutkimuslaitos & Kustannusosakeyhtiö Metsälehti. p. 141–152.
Tarrant R.F., Trappe J.H. (1971). The role of Alnus improving the forest environment. Plant and Soil (Special volume): 335–348.
Turner J., Cole D.W., Gessel S.P. (1976). Mineral nutrient accumulation and cycling in a stand of red alder (Alnus rubra). Journal of Ecology 64: 965–974.
Turunen J. (1953). Huomioita harmaalepän juuristosta ja juuriäkämistä. Metsätaloudellinen aikakauslehti 83(9): 362–366.
Uri V., Aosaar J., Varik M., Becker H., Ligi K., Padari A., Kanal A., Lõhmus K. (2014). The dynamics of biomass production, carbon and nitrogen accumulation in grey alder (Alnus incana (L.) Moench) chronosequence stands in Estonia. Forest Ecology and Management 327: 106–117. http://dx.doi.org/10.1016/j.foreco.2014.04.040.
Uri V., Tullus H., Lõhmus K. (2002). Biomass production and nutrient accumulation in short-rotation grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forest Ecology and Management 161(1–3): 169–179. http://dx.doi.org/10.1016/S0378-1127(01)00478-9.
Uri V., Tullus H., Lõhmus K. (2003). Nutrient allocation, accumulation and above-ground biomass in grey alder and hybrid alder plantations. Silva Fennica 37(3): 301–11. http://dx.doi.org/10.14214/sf.490.
Uri V., Lõhmus K., Mander U., Ostonen I., Aosaar J., Maddison M., Helmisaari, H-S., Augustin J. (2011). Long-term effects on the nitrogen budget of a short-rotation grey alder (Alnus incana (L.) Moench) forest on abandoned agricultural land. Ecological Engineering 37(6): 920–930. http://dx.doi.org/10.1016/j.ecoleng.2011.01.016.
Viro P.J. (1955). Investigations on forest litter. Communicationes Instituti Forestalis Fenniae 67(7). 49 p.
Virtanen A.J. (1957). Investigations on nitrogen fixation by the alder II. Associated culture of spruce and inoculated alder without combined nitrogen. Physiologia Plantarum 10: 164–169.
Zavitkovski J.R., Newton M. (1968). Effect of organic matter and combined nitrogen on nodulation and nitrogen fixation in red alder. In: Trappe J.M., Franklin J.F., Hansen G.M. (eds.). Biology of alder. USDA Forest Service, PNW Forest and Range Experimental Station, Portland, OR. p. 209–223.
Zimka J.R., Stachurski A. (1976). Vegetation as a modifier of carbon and nitrogen transfer to soil in various types of forest ecosystems. Ekologia Polska 24: 493–514.
Total of 59 references


