Antennal response of Prinobius myardi to synthetic tree volatiles
Sánchez-Osorio I., Domínguez L., López-Pantoja G., Tapias R. (2015). Antennal response of Prinobius myardi to synthetic tree volatiles. Silva Fennica vol. 49 no. 3 article id 1305. https://doi.org/10.14214/sf.1305
Highlights
- Prinobius myardi is a wood borer considered a major threat for Mediterranean oaks, especially Quercus suber and Q. ilex
- We performed electroantennographic bioassays to assess olfactory sensitivity of P. myardi to synthetic plant volatiles
- P. myardi exhibits a broad sensitivity to common tree volatiles, including those emitted by oaks (α-pinene and β-pinene) or non-host volatiles (1,8-cineole).
Abstract
Prinobius myardi Mulsant is a wood borer implicated in the decline of Mediterranean oaks, especially Quercus suber L. and Quercus ilex L. Plant volatiles play an important role in plant-insect interactions, and electroantennography (EAG) is an effective tool for exploring the electrophysiological activity of host plant volatiles on insects. To improve our understanding of the olfactory sensitivity of P. myardi, we recorded EAG responses to 20 tree volatiles, and analyzed the dose-dependent response to five doses (10–4:1 to 1:1 v/v) of the three most EAG-active compounds. Antennae of P. myardi responded to 13 chemicals, mainly monoterpenes and green leaf volatiles, with the strongest EAG responses being observed with β-pinene, (+)-α-pinene and 1,8-cineole. Dose–response profiles showed positive dose-dependent responses for all three compounds. Our results suggest a broad sensitivity of P. myardi to common tree volatiles, particularly some host-related compounds and volatiles associated with wounded trees; the olfactory recognition of ratios of these compounds could play a role in host selection by P. myardi.
Keywords
Quercus;
EAG;
electroantennography;
wood borers;
Cerambycidae;
plant volatiles;
β-pinene
-
Sánchez-Osorio,
Departamento de Ciencias Agroforestales, ETSI La Rábida, University of Huelva, 21819 Palos de la Frontera (Huelva), Spain
 http://orcid.org/0000-0002-6852-7699
E-mail
isanchez@uhu.es
http://orcid.org/0000-0002-6852-7699
E-mail
isanchez@uhu.es

-
Domínguez,
Departamento de Ciencias Agroforestales, ETSI La Rábida, University of Huelva, 21819 Palos de la Frontera (Huelva), Spain
 http://orcid.org/0000-0002-0131-0057
E-mail
luis.dominguez@dcaf.uhu.es
http://orcid.org/0000-0002-0131-0057
E-mail
luis.dominguez@dcaf.uhu.es
-
López-Pantoja,
Departamento de Ciencias Agroforestales, ETSI La Rábida, University of Huelva, 21819 Palos de la Frontera (Huelva), Spain
 http://orcid.org/0000-0002-2659-6127
E-mail
pantoja@uhu.es
http://orcid.org/0000-0002-2659-6127
E-mail
pantoja@uhu.es
- Tapias, Departamento de Ciencias Agroforestales, ETSI La Rábida, University of Huelva, 21819 Palos de la Frontera (Huelva), Spain E-mail rtapias@uhu.es
Received 22 January 2015 Accepted 29 April 2015 Published 15 May 2015
Views 89040
Available at https://doi.org/10.14214/sf.1305 | Download PDF

1 Introduction
Wood-boring insects play a significant role in oak decline in the Mediterranean area (Sallé et al. 2014). As they colonize weakened trees, they act as an aggravating factor, hindering tree recovery (López-Pantoja et al. 2011). Mediterranean Quercus ilex L. and Quercus suber L. dehesa open woodlands of the Andalucía region (Southern Spain) have high ecological, economic and cultural importance. Hence, problems affecting stands of these species, including invasion by wood-boring beetles such as Prinobius myardi Mulsant (Coleoptera, Cerambycidae), deserve particular attention especially in a context of climate change (López-Pantoja et al. 2011; Sallé et al. 2014).
The importance of volatile semiochemicals in host selection by Cerambycidae species has mostly been studied for species attacking conifers (see Allison et al. 2004); and relatively little is known about the role of such compounds in host location by wood borers infesting deciduous trees – especially Quercus species – though notable results have been obtained for Cerambyx welensii Küster (Torres-Vila et al. 2012 and 2013; Sánchez-Osorio et al. 2015). Electroantennography (EAG) is an effective tool for measuring the total response of insect antennal receptor cells to olfactory stimuli (Bruce et al. 2005; Gullan and Cranston 2014), and has already been applied to several cerambycid wood borers, including C. welensii and P. myardi (Sánchez-Osorio et al. 2007 and 2009), Saperda populnea L. (Chi et al. 2011) and Batocera lineolata Chevrolat (Yang et al. 2013).
Prinobius myardi is a large (up to 60 mm long), holomediterranean species with nocturnal habits, whose larvae bore into wood of several deciduous tree species, especially weakened cork and holm oaks, causing tree branches and trunks to break (López-Pantoja et al. 2011). In addition, these insects facilitate infection by both plant pathogens and wood-decaying fungi (Martin et al. 2005). Despite these effects, several aspects of the behavior of P. myardi are not well known, in particular, regarding its role in oak decline, host selection cues and the development of control methods (López-Pantoja et al. 2011; Torres-Vila et al. 2013).
Quercus suber and Q. ilex are strong emitters of foliar monoterpenes, mainly α-pinene, β-pinene, limonene, sabinene and myrcene (Staudt et al. 2008; Loreto et al. 2014). Several green leaf volatiles (GLVs), in particular (E)-2-hexenal, (Z)-3-hexenyl acetate and (E)-2-hexenol, are also emitted by freshly cut branches and leaf extracts of Q. suber (Fürstenau et al. 2012). Recently, it has been suggested that colonization of Q. suber trees by C. welensii is positively associated with foliar emission of limonene (Sánchez-Osorio et al. 2008).
To understand the olfactory sensitivity of P. myardi to plant volatiles, we examined: (a) the EAG responses of P. myardi to individual synthetic tree volatiles; and (b) the dose–response relationships for the three most EAG-active compounds.
2 Material and methods
2.1 Test substances
The odorant test panel comprised 20 synthetic chemicals. The main group included 16 analogues of volatiles identified in foliar emissions from Q. suber and Q. ilex: the monoterpenes, β-pinene, (+)-α-pinene, (+)-limonene, β-myrcene, 1,8-cineole, (E)-β-ocimene, α-terpinene, γ-terpinene, α-phellandrene, ρ-cymene and α-terpineol (Sánchez-Osorio et al. 2008; Llusià et al. 2012); the GLVs, (E)-2-hexenal, (Z)-3-hexenol and (Z)-3-hexenyl acetate (Fürstenau et al. 2012); and the triterpenes, lupenone and erithrodiol (Monaco and Previtera 1984). We also tested several analogues of compounds found in Q. suber cork, namely gallic and ellagic acids, the triterpene friedelin and 3-methyl-butanol (Rocha et al. 1996). Turpentine oil was used as the standard stimulus (Sánchez-Osorio et al. 2007). All chemicals were purchased from Sigma-Aldrich (Madrid, Spain). Purity was ≥ 94%, except in the cases of ρ-cymene and turpentine, which had > 90% purity.
2.2 Electroantennography
Prinobius myardi adults were collected by hand picking during the flight period (July, 2004) in a Q. suber dehesa (Huelva, SW Spain). Insects (3.8 ± 0.4 cm long, 1.2 ± 0.3 g weight; on average) were kept in individual containers with access to a 4% sugar solution, in semi-darkness (at 23–32 °C). Notably, P. myardi females were difficult to capture (the average female:male capture ratio being 0.28 over eight years (López-Pantoja et al. 2011)), and most of those collected died within 24 hours.
EAG responses were recorded 1 to 7 days (mean of 4 days) after insect collection, as in Sánchez-Osorio et al. (2007), using glass capillary electrodes (0.5-mm internal diameter) filled with saline solution (KCL 0.1 N, with 3% polyvinylpyrrolidone) and slipped over silver wire. The distal end of the antenna was inserted into the tip of the recording electrode; and the indifferent electrode (same glass capillary, custom-pulled to an approximately 100-µm tip diameter) was inserted through the membrane between scape and pedicel. Each chemical compound tested was dissolved in hexane (Sigma-Aldrich, 99% purity) to prepare odor sources, and 20-µl aliquots of these solutions were applied to filter paper strips (Whatman No 1). The solvent was allowed to evaporate for 5 min before each paper strip was inserted into a glass Pasteur pipette. For stimulation, 10-ml puffs of air were blown through the Pasteur pipettes into a purified air stream continuously flowing over the antenna (4 l min–1), using a Windjet 727-RY-15 diffuser (Spraying Systems GmbH, Hamburg, Germany) to ensure covering the entire antenna length. EAG responses were recorded (in mV) using Syntech electrode holders, IDAC-4 acquisition controller and Autospike-32 software (Syntech, Hilversum, The Netherlands).
In Experiment 1, EAG responses were recorded in five stimulation series of four randomly-puffed individual odor sources (1:1 v/v dilution of each compound in hexane). Each series was preceded by a stimulus with turpentine standard as a reference for EAG response over the session; a last turpentine stimulus was also applied 10 min after the last series. There was a 10-min interval between series, and 1-min interval between stimulations within a series. The same seven insects (five males and two females) were tested with all the compounds (one replication per individual and per compound).
In Experiment 2, the three compounds eliciting the strongest EAG responses in Experiment 1 – β-pinene, (+)-α-pinene and 1,8-cineole – were presented at five doses increasing by orders of magnitude from 10–4:1 (corresponding to 858–923 ηg depending on the chemical) to 1:1 v/v in hexane. For each chemical, dose–response was tested in five stimulation series with three replicates for each dose, preceded by a stimulus with turpentine standard. In this case, there were 1-min intervals between series, and between replicates within series; a last turpentine stimulus was applied 1 min after the last series, and 10-min intervals separated tests with different compounds. We calculated the mean response from the three replicates of a specific dose to minimize variation associated with manual injections. The dose–response was tested for each of the three chemicals using the same five insects (three males and two females, different individuals from those used for experiment 1).
Measured EAG responses were corrected by subtracting the mean response to two hexane control injections (20 µl) recorded at the beginning and end of each series in both experiments, to compensate for solvent and/or mechanoreceptive artifacts. Corrected measures were then normalized to percentages relative to the EAG response obtained to the standard stimuli, namely, the mean of the EAG responses to the two turpentine injections between which the response to each chemical or specific dose was recorded (Yang et al. 2013).
2.3 Data analysis
Grubbs’s tests showed outliers in male EAG responses, probably due to the heterogeneous vitality of field-caught insects, and hence, normality and homoscedasticity assumptions were not met. In such cases, robust statistical methods have been recommended (Wilcox 2012). EAG responses were compared using functions implemented in the WRS package (Wilcox 2012), based on 20% trimmed means (Mean0.2: mean calculated after discarding the top and bottom 20% of values). Two-sided confidence intervals for the difference in Mean0.2 EAG responses revealed no differences between sexes for any of the tested compounds (P > 0.1), and hence, EAG data from males and females were pooled.
Data from both experiments were analyzed using heteroscedastic ANOVA for related samples (two-way analysis for Experiment 2), followed by Rom’s modified Bonferroni tests for multiple comparisons. One-sided confidence intervals based on the Mean0.2 for related samples were used to assess whether normalized (uncorrected) EAG responses to individual compounds were larger than the response to the hexane control. All statistical analyses were performed using the R framework (R Development Core Team 2014, version 3.1.1), with α = 0.05, employing corrected (except when comparing to hexane controls) and then normalized EAG values.
3 Results
EAG signals showed the typical rapid depolarization followed by a slower recovery phase. The EAG response to standard turpentine was weak (0.38 ± 0.17 mV, Mean0.2 ± Error0.2, N = 7) and to the hexane control was negligible (Mean0.2 < 0.01 mV, N = 7). Compared to the hexane control, EAG responses were significantly higher for 13 compounds: all tested monoterpenes except ρ-cymene and α-terpineol, the three GLVs and 3-methyl-butanol.
In Experiment 1 (Fig. 1), there were significant differences in EAG responses to individual compounds (ANOVA: F6 24.1 = 14.6, P < 0.001). Three compounds, β-pinene, 1,8-cineole and (+)-α-pinene, clearly elicited the highest responses, with no significant differences between them. Stimulation with the other 10 active compounds produced moderate-intensity EAG signals (normalized response range: 35–48%), with six compounds being significantly stronger stimulants than ρ-cymene: α-terpinene, (E)-β-ocimene, β-myrcene, (E)-2-hexenal, α-phellandrene and (Z)-3-hexenol.
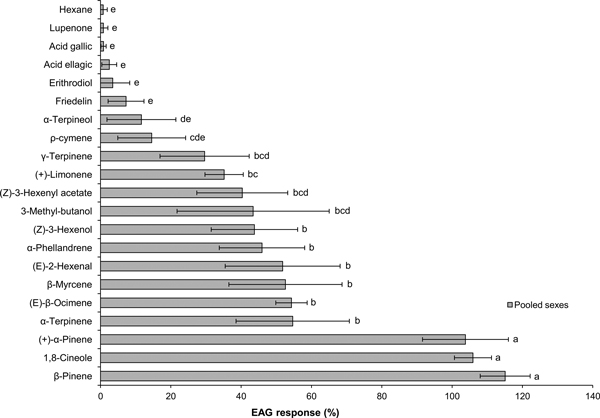
Fig. 1. Mean0.2 (± Error0.2) EAG responses of Prinobius myardi to tree volatiles. Responses are expressed as normalized percentages relative to the standard reference (turpentine oil), after subtracting the response to the hexane control. The hexane control response is included for comparison. Bars with the same letter are not significantly different (heteroscedastic ANOVA for related samples, N = 7).
In Experiment 2, responses to the three strongest stimulants was significantly dose dependent (Fig. 2), with no significant dose and compound interaction (ANOVA: P < 0.001, P = 0.462, and P = 0.181; respectively for dose, compound, dose × compound). No or negligible EAG responses were elicited by 10–2:1 and lower doses. Dose–response profiles did not indicate that any saturation threshold had been reached; however, a 1:1 dose of 1,8-cineole did not elicit a significantly higher EAG response than a 10–1:1 dose (P = 0.160).
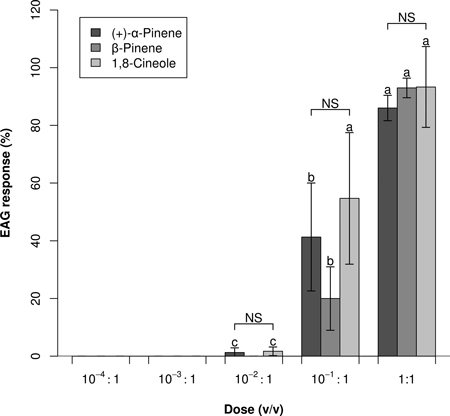
Fig. 2. Dose–response EAG profiles (Mean0.2 ± Error0.2) of Prinobius myardi. Responses are expressed as normalized percentages relative to the standard reference turpentine oil (not shown). For each compound, letters on the columns denote differences between doses; NS indicates no significant difference between compounds at each dose (heteroscedastic ANOVA for related samples, N = 5).
4 Discussion
Prinobius myardi shows olfactory sensitivity to common tree volatiles, particularly some volatiles emitted by host species. Host location by insects involves recognition of specific semiochemicals or, more often, specific mixtures of common plant volatiles (Allison et al. 2004; Bruce et al. 2005; Smart et al. 2013). Quercus suber and Q. ilex, the main hosts of P. myardi, are considered strong monoterpene emitters, with four leaf chemotypes which are genetically based: a pinene type, a limonene type, and either an intermediate limonene/pinene type (in Q. suber) or a myrcene type (in Q. ilex) (Staudt et al. 2008; Loreto et al. 2014). Two monoterpenes widely emitted by trees (especially from resinous species), β-pinene and (+)-α-pinene, together with 1,8-cineole, elicited the highest EAG responses in P. myardi, and these were dose-dependent. Limonene and myrcene elicited lower EAG responses than the two pinene isomers, suggesting P. myardi is able to discriminate between leaf chemotypes, particularly pinene from both limonene and myrcene types.
The mediation of plant volatiles in host location has been reported within Cerambycidae (Allison et al. 2004; Hanks et al. 2012). Sánchez-Osorio et al. (2008) found limonene emission from Q. suber leaves to be higher in trees heavily colonized by C. welensii than neighboring non-colonized trees. Further, (+)-Limonene and two related compounds (α-phellandrene and α-terpinene) elicited moderate EAG responses in P. myardi, high concentrations of α-phellandrene having been found in leaf emissions from Q. ilex (Llusià et al. 2012). Plant volatiles are also probably involved in the recognition of weakened, damaged hosts (Allison et al. 2004; Hanks et al. 2012). Moderate EAG responses were elicited in P. myardi by three compounds associated with emissions from wounded leaves of Quercus species: the known ubiquitous GLV (E)-2-hexenal, two acyclic monoterpenes, β-myrcene and (E)-β-ocimene (Paris et al. 2011; Pearse et al. 2013), and 3-methyl-butanol (found in microbially colonized cork from Q. suber (Rocha et al. 1996)).
Using olfactory receptor neurons for common plant compounds allows insects to evaluate greater ranges of potential hosts (Bruce et al. 2005). It is not possible to directly match EAG responses to a behavioral activity (e.g., attraction or repulsion); and moreover, the heterogeneous vitality of the insects (survival after EAG test: 3–18 days in males; 1–2 days for females) and the small sample could have biased EAG responses. However, our results allow us to formulate a tentative two-stage hypothesis concerning reliance on common tree volatiles in host location by P. myardi, which should be further explored. At a first stage, the specific recognition of strong EAG-active compounds – β-pinene, (+)-α-pinene and 1,8-cineole – could help distinguish suitable from unsuitable hosts at patch scale (e.g., avoiding non-host Pinus and Eucalyptus genera, which include 1,8-cineole as a major terpenic component. Barata et al. 2000; Blanch et al. 2007) and possibly also within patches, enabling P. myardi to recognize trees with pinene chemotype (and, in turn, this could influence the probability of conspecific encounters, as suggested for C. welensii based on Q. suber leaf chemotypes). At a second stage, P. myardi could use specific ratios of several compounds eliciting moderate EAG responses to identify blends of volatiles characterizing host species, even at the intraspecific level (e.g., relative fractions of limonene and myrcene in terpenic emission from Q. ilex and Q. suber), or wounded trees (e.g., some GLVs, (E)-β-ocimene or 3-methyl-butanol).
Behaviorally-active semiochemicals, such as host plant volatiles, have potential applications in integrated pest management including for monitoring and mass-trapping programs (Allison et al. 2004; Smart et al. 2013). Our results are promising as a basis for such applications in P. myardi, especially in the context of declining oak populations, as is the case for Q. suber and Q. ilex dehesas. However, there is a need for further studies focused on improving electrophysiological testing (especially with females) for tree volatiles, as well as performing behavioral bioassays (field trials with baited traps, and flight tunnels) to thoroughly investigate activity induced by EAG-active compounds.
Acknowledgements
This work was funded by the Andalusian Regional Council of Environment and Spatial Planning.
References
Allison J., Borden J., Seybold J. (2004). A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14: 123–150. http://dx.doi.org/10.1007/s00049-004-0277-1.
Barata E.N., Pickett J.A., Wadhams L.J., Woodcock C.M., Mustaparta H. (2000). Identification of host and nonhost semiochemicals of Eucalyptus woodborer Phoracantha semipunctata by gas chromatography–electroantennography. Journal of Chemical Ecology 26: 1877–1895. http://dx.doi.org/10.1023/A:1005548824429.
Blanch J.S., Peñuelas J., Llusià J. (2007). Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiologia Plantarum 2: 211–225. http://dx.doi.org/10.1111/j.1399-3054.2007.00944.x.
Bruce T.J.A., Wadhams L.J., Woodcock C.M. (2005). Insect host location: a volatile situation. Trends in Plant Science 10: 269–274. http://dx.doi.org/10.1016/j.tplants.2005.04.003.
Chi D., Li X., Yu J., Xie X., Wang G. (2011.) The EAG response and behavior of the Saperda populnea L. to volatiles from poplar branches. Acta Ecologica Sinica 31: 334–340. http://dx.doi.org/10.1016/j.chnaes.2011.09.003.
Fürstenau B., Rosell G., Guerrero A., Quero C. (2012). Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. Journal of Chemical Ecology 38: 378–388. http://dx.doi.org/10.1007/s10886-012-0110-1.
Gullan P.J., Cranston P.S. (2014). The insects. An outline of entomology. Fifth edition. John Wiley & Sons Ltd, Chichester. 624 p.
Hanks L.M., Millar J.G., Mongold-Diers J.A., Wong J.C.H., Meier L.R., Reagel P.F., Mitchell R.F. (2012). Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Canadian Journal of Forest Research 42: 1050–1059. http://dx.doi.org/10.1139/x2012-062.
Llusià J., Peñuelas J., Seco R., Filella I. (2012). Seasonal changes in the daily emission rates of terpenes by Quercus ilex and the atmospheric concentrations of terpenes in the Natural Park of Montseny, NE Spain. Journal of Atmospheric Chemistry 69: 215–230. http://dx.doi.org/10.1007/s10874-012-9238-1.
López-Pantoja G., Domínguez Nevado L., Sánchez-Osorio I. (2011). Analysis of Prinobius myardi Mulsant population dynamics in a Mediterranean cork oak stand. Annales de la Société Entomologique de France 47: 260-268. http://dx.doi.org/10.1080/00379271.2011.10697717.
Loreto F., Pollastri S., Fineschi S., Velikova V. (2014). Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Journal Environmental and Experimental Botany 103: 99–106. http://dx.doi.org/10.1016/j.envexpbot.2013.09.005.
Martin J., Cabezas J., Buyolo T., Patón D. (2005). The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. Forest Ecology and Management 216: 166–174. http://dx.doi.org/10.1016/j.foreco.2005.05.027.
Monaco P., Previtera L. (1984). Isoprenoids from the leaves of Quercus suber. Journal of Natural Products 47: 673–676. http://dx.doi.org/10.1021/np50034a017.
Paris C.I., Llusia J., Peñuelas J. (2011). Indirect effects of tending ants on holm oak volatiles and acorn quality. Plant Signaling & Behavior 6: 547–550. http://dx.doi.org/10.4161/psb.6.4.14839.
Pearse I.S., Gee W.S., Beck J.J. (2013). Headspace volatiles from 52 oak species advertise induction, species identity, and evolution, but not defense. Journal of Chemical Ecology 39: 90–100. http://dx.doi.org/10.1007/s10886-012-0224-5.
R Development Core Team (2014). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rocha S., Delgadillo I., Correia A. (1996). GC-MS study of volatiles of normal and microbiologically attacked cork from Quercus suber L. Journal of Agricultural and Food Chemistry 44: 865–871. http://dx.doi.org/10.1021/jf9500400.
Sallé A., Nageleisen L.-M., Lieutier F. (2014). Bark and wood boring insects involved in oak declines in Europe: current knowledge and future prospects in a context of climate change. Forest Ecology and Management 328: 79–93. http://dx.doi.org/10.1016/j.foreco.2014.05.027.
Sánchez-Osorio I., Tapias R., Domínguez L., López G. (2007). Caracterización de la respuesta electroantenográfica de Cerambyx welensii Küster y Prinobius germari Dejean (Coleoptera, Cerambycidae). Investigación Agraria. Sistemas y Recursos Forestales [currently Forest Systems] 16: 95–106. http://dx.doi.org/10.5424/srf/2007161-01001.
Sánchez-Osorio I., Domínguez L., López G., Tapias R., Sánchez-Rodas D. (2008). Perfil de emisión de compuestos orgánicos volátiles (COV) foliares en alcornoques infestados por Cerambyx welensii Küster (Coleoptera: Cerambycidae). Cuadernos de la Sociedad Española de Ciencias Forestales 26: 59–65.
Sánchez-Osorio I., Tapias R., Domínguez L., López-Pantoja G. (2009). Variabilidad intraespecífica de la respuesta electroantenográfica en Cerambyx welensii Küster (Coleoptera, Cerambycidae), influencia de factores anatómicos, fisiológicos y experimentales. Investigación Agraria. Sistemas y Recursos Forestales [currently Forest Systems] 18: 140–151.
Sánchez-Osorio I., López G., Paramio A.M., Lencina J.L., Gallego D., Domínguez L. (2015). Field attraction of Cerambyx welensii to fermentation odors and host monoterpenes. Journal of Pest Science. http://dx.doi.org/10.1007/s10340-015-0654-2.
Smart L.E., Aradottir G.I., Bruce T.J.A. (2013). Role of semiochemicals in integrated pest management. In: Abrol D. (ed.). Integrated pest management. Current concepts and ecological perspective. Elsevier, London. p. 93–109.
Staudt M., Ennajah A., Mouillot F., Joffre R. (2008). Do volatile organic compound emissions of Tunisian cork oak (Quercus suber) populations originating from contrasting climatic conditions differ in their responses to summer drought? Canadian Journal of Forest Research 33: 870–884.
Torres-Vila L.M., Sánchez-González Á., Ponce-Escudero F., Martín-Vertedor D., Ferrero-García J.J. (2012). Assessing mass trapping efficiency and population density of Cerambyx welensii Küster by mark-recapture in dehesa open woodlands. European Journal of Forest Research 131: 1103–1116. http://dx.doi.org/10.1007/s10342-011-0579-0.
Torres-Vila L.M., Sánchez-González Á., Merino-Martínez J., Ponce-Escudero F., Conejo-Rodríguez Y., Martín-Vertedor D., Ferrero-García J.J. (2013). Mark-recapture of Cerambyx welensii in dehesa woodlands: dispersal behaviour, population density, and mass trapping efficiency with low trap densities. Entomologia Experimentalis et Applicata 149: 273–281. http://dx.doi.org/10.1111/eea.12133.
Wilcox R.R. (2012). Introduction to robust estimation and hypothesis testing. Academic Press, San Diego. 690 p.
Yang H., Yang W., Yang C.-P., Zhu T.-H., Huang Q., Han S., Xiao J.-J. (2013). Electrophysiological and behavioral responses of the whitestriped longhorned beetle, Batocera lineolata, to the diurnal rhythm of host plant volatiles of holly, Viburnum awabuki. Journal of Insect Science 13: 1–11. http://dx.doi.org/10.1673/031.013.8501.
Total of 28 references

