Significance of the root connection on the dormancy release and vegetative bud burst of Norway spruce (Picea abies) seedlings in relation to accumulated chilling
Partanen J., Häkkinen R., Hänninen H. (2015). Significance of the root connection on the dormancy release and vegetative bud burst of Norway spruce (Picea abies) seedlings in relation to accumulated chilling. Silva Fennica vol. 50 no. 1 article id 1443. https://doi.org/10.14214/sf.1443
Highlights
- Cutting the root connection slightly increased the number of days to bud burst of Norway spruce seedlings under warm conditions but it had no consistent effect on bud burst percentage
- Our results obtained with seedlings suggest that using detached tree material in dormancy release experiments may slightly affect the results but it will evidently not lead to drastically erroneous conclusions.
Abstract
The effect of cutting the root connection by detaching the shoot from the root system on dormancy release and vegetative bud burst was examined in 2-year-old seedlings of Norway spruce (Picea abies [L.] Karst.). Seedlings were transferred at 1–4 week intervals between October and January from outdoor conditions to experimental forcing in a heated greenhouse. Before forcing, half of the seedlings were cut above ground line, and the detached shoots were forced with their cut ends placed in water. The intact seedlings were forced with their root system remaining intact in the pots. Vegetative bud burst was observed visually. Cutting the root connection slightly increased days to bud burst in the forcing conditions, however, no consistent effect on bud burst percentage was found. Our preliminary seedling data suggest that using detached tree material in dormancy release experiments may have a small effect on bud burst date but it will evidently not lead to drastically erroneous conclusions. Further studies, using different seed lots, are needed to assess the effect of detaching on the dormancy release and bud burst, especially in adult trees.
Keywords
phenology;
bud burst percentage;
days to bud burst;
dormancy release;
forcing
-
Partanen,
Natural Resources Institute Finland (Luke), Management and Production of Renewable Resources, Juntintie 154, FI-77600 Suonenjoki, Finland
E-mail
jouni.partanen@luke.fi

- Häkkinen, Natural Resources Institute Finland (Luke), Management and Production of Renewable Resources, P.O. Box 18, FI-01301 Vantaa, Finland E-mail risto.hakkinen@luke.fi
- Hänninen, University of Helsinki, Department of Biosciences, Viikki Plant Science Centre (ViPS), P.O. Box 65, FI-00014 University of Helsinki, Finland E-mail heikki.hanninen@helsinki.fi
Received 26 August 2015 Accepted 20 November 2015 Published 4 December 2015
Views 87521
Available at https://doi.org/10.14214/sf.1443 | Download PDF

1 Introduction
In the northern latitudes, where seasonal variation in temperature and light conditions is strong, photoperiod primarily controls the induction of dormancy of woody plants at the end of summer (Garner and Allard 1923; Wareing 1956). Several weeks after growth cessation in autumn, the buds enter endodormancy, where the growth of the buds is constrained even under favourable growing conditions (Lang et al. 1987). Release from bud endodormancy requires exposure to low chilling temperatures (e.g. –5 to +10 °C) during autumn (Coville 1920; Nienstaedt 1966, 1967; Sarvas 1974). The release of endodormancy indicates the gradual achieving of ontogenetic competence, i.e., ability to grow in favourable conditions (Hänninen 1995; Hänninen and Kramer 2007; Junttila and Hänninen 2012).
After endodormancy the buds are in a state of ecodormancy, i.e., they have full ontogenetic competence but still remain dormant due to prevailing low air temperature. Ecodormancy is released by forcing temperatures (e.g., T > +5 °C), and bud burst occurs when accumulated temperature sum exceeds a specific threshold (Sarvas 1972; Campbell and Sugano 1975; Hannerz 1994; Sutinen et al. 2012). The release from bud endodormancy, the transition to ecodormancy, and the final transition to bud burst are critical for the seedlings and trees growing in boreal and temperate regions. If the endodormancy is released prematurely, growth initiation during warm spells in autumn and winter could expose them to frost injury during subsequent low temperatures.
Most studies on dormancy release and bud burst have been done using young seedlings (e.g. Nienstaedt 1967; Hänninen 1990). According to these studies, release of endodormancy occurs in Finnish conditions in general during late autumn, say in October–December (Hänninen and Pelkonen 1989; Hänninen 1990; Hänninen and Backman 1994). However, it is not clear whether the experimental results obtained with seedlings can be generalized to adult trees, because the overall physiology of the trees changes according to their age. The change in seedling physiology from free growth pattern to predetermined growth occurs in Norway spruce (Picea abies [L.] Karst.) at the age of 5–10 years (Jablanczy 1971). It may involve a change in the response factors controlling dormancy release and bud burst (Søgaard et al. 2008).
Experimental studies on dormancy release and bud burst of adult trees are technically difficult to conduct, because it is practically impossible to transfer adult trees to the greenhouse. Therefore, in addition to young seedlings, branches detached from the trees have been commonly used in forcing experiments. Partanen et al. (2005) for instance found that the bud burst percentage of detached twigs of Norway spruce trees varies during winter, and depends on the age of the tree. However, detaching the twig may affect its physiology. In addition to possibly disturbing the intake of water, detaching disconnects the branches from possible growth promoting or inhibiting signals of bud burst, such as phytohormones. Abscisic acid is known to maintain, and gibberellic acid (GA) and cytokinins to release bud endodormancy (Wareing and Saunders 1971; Arora et al. 2003; Chao et al. 2007; Cooke et al. 2012). However, in winter the maintenance and the release of endodormancy might reside merely within the buds, which could respond autonomously to environmental cues, such as specific combinations of low and moderate temperatures, or specific photoperiods (Lang 1994).
In recent studies, contradictory results concerning the potential effects of the detaching of the twigs have been obtained. Detached branches from the adult trees have been suggested to be more conservative than intact whole trees in spring phenology (Basler and Körner 2012). On the other hand, detached twigs from adult trees have been recently shown to be a good proxy to explore phenological responses of adult deciduous trees in temperate forests (Vitasse and Basler 2014).
Though the dormancy physiology and bud burst phenology of seedlings may differ from those in adult trees, the effect of detaching of the twigs on bud burst can be approximated using both intact and cut seedlings in experiments. Our aim was to study the effect of cutting the root connection by detaching the shoot from the root system on dormancy release in 2-year-old seedlings of Norway spruce. To this end the bud burst of both of the cut seedlings and of the intact seedlings was observed in a heated greenhouse after chilling of varying duration in natural conditions.
2 Material and methods
The 2-year-old seedlings used in the experiments were grown at the Finnish Forest Research Institute (currently Natural Resources Institute Finland) in Suonenjoki (62°39´N, 27°03´E, altitude 142 m a.s.l.). The seedlings were grown in hard-plastic containers Plantek PL64F (64 cells per tray, 432 cells m–2, cell volume 110 cm3; BCC, Landskrona, Sweden) filled with base-fertilized light sphagnum peat (Kekkilä M6W; Kekkilä Oyj, Eurajoki, Finland). The seed had been collected from the natural stands in Virrat, Maalahti, Korsnäs and Längelmäki (61°32´N – 63°02´N; 21°05´E – 24°59´E, altitude 84–225 m a.s.l.). The seeds were sown between 31 May and 2 June 2006, and the seedlings were raised according to standard nursery practice in Finland. The seedlings were given 180 g/m2 a commercial fertilizer solution (Kekkilä Taimi-Superex) (19N:4P:20K + micronutrients; Kekkilä Co., Tuusula, Finland). During the second growing season the seedlings were subjected to a short-day (12 h) treatment between 13 and 24 August 2007.
The seedlings were transferred to Punkaharju (the Finnish Forest Research Institute; 61°48´N, 29°19´E) on 11 September 2007. They were transplanted to Soparco 7 × 7 × 8 cm plastic pots filled with Kekkilä M6 peat (Kekkilä Oyj, Eurajoki, Finland) on 19 and 20 September. The seedling pots were put in to polystyrene boxes to shelter the roots during winter. On 12 November seedling boxes were moved to a partially open greenhouse to shelter seedlings from snow and provide easier winter access to the test population. Air temperature recorded using Tinytalk Data Loggers (Gemini Data Loggers, Chichester, UK) in the unheated greenhouse and outside was very similar.
The seedlings were transferred from outdoor chilling conditions at 1- to 4-week intervals between 2 October 2007 and 22 January 2008. At each of the 12 transfers, 40 seedlings were transferred. The transfer dates from outdoor conditions were 2, 16, 23, 30 October; 6, 13, 20, 27 November; 4, 11 December and 8, 22 January. Until 30 October, the seedlings were transferred directly to forcing conditions in the greenhouse, where the air temperature was kept constant at +20 °C, and the day length was 12 h (cf. Søgaard et al. 2008). From 6 November on, when the outdoor temperature fell far below 0°C, the seedlings were first transferred to preforcing conditions (darkness, +1 to +4 °C temperature) for one week to enable cautious thawing and to prevent needle damages (Repo et al. 1984), and then moved to forcing conditions. In the presentation of the results the preforcing is accounted for so that the day of transfer from the preforcing to the forcing conditions is taken as the transfer day.
The photoperiod in the forcing conditions was controlled using black curtains preventing light to reach the seedlings. The natural day length was extended using metal halide lamps (Philips HPI-T Powertone 400 W). Photosynthetically active radiation at the shoot level was approximately 50 µE m–2 s–1. The curtains were kept open, and the lamps were on between 6 am and 6 pm (standard time). The air temperature was recorded using Tinytalk Data Loggers (Gemini Data Loggers, Chichester, UK) once an hour at the shoot level in the outdoor chilling and indoor preforcing and forcing conditions. The average daily air temperatures and the chilling unit sum (ch.u.) in the outdoor and preforcing conditions are presented in Fig. 1. The chilling unit sum was calculated on the basis of hourly temperature measurements, using the tabulated air temperature response values (T –3.5 to +10.5 °C, maximum accumulation rate at T +3.4 to +3.6 °C) of Sarvas (1974). The temperature sums in forcing conditions were calculated from the daily mean temperatures (T ≥ +5 °C).
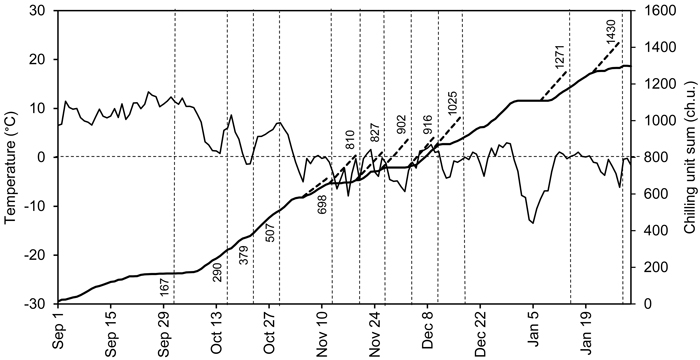
Fig. 1. Average daily air temperatures (solid fluctuating curve) and the chilling unit sum based on hourly temperature measurements in outdoor conditions (solid ascending curve) at Punkaharju, Finland (61°48´N, 29°19´E), from September 1, 2007 to January 31, 2008. Dashed vertical lines indicate the transfers, i.e., the time points at which the seedlings were moved to forcing conditions. The attached figures indicate the corresponding accumulated chilling unit sums. The dashed ascending lines indicate the chilling units that accumulated in the preforcing conditions for one week. The dashed horizontal line indicates a temperature of 0 °C and chilling unit sum of 800 ch.u.
Before forcing, 20 seedlings out of 40 were cut at ground line. The detached shoots of these cut seedlings were kept with their cut ends placed in water. To guarantee a water supply to the detached shoots, their ends were cut once a week when the water in the buckets was changed. Moreover, water was automatically sprinkled on the shoots to keep them moist. The intact seedlings were forced with their root system remaining intact in the pots. They were watered manually.
Vegetative bud burst was observed visually three times a week at intervals of two or three days until bud burst had taken place or the observed buds were visibly dead. The terminal bud of the shoot and the tip buds of the four uppermost lateral shoots, which were not always in the same whorl, were observed. A bud was considered to have burst when new needles were visible. The bud burst percentages, BB%, of intact and cut seedlings in each transfer were calculated separately for terminal buds and for observed lateral buds (four per seedling). Accordingly, average days to bud burst, DBB, were calculated separately as the average time from the beginning of forcing to the day of the terminal bud burst and to the day when at least two of the four observed lateral buds of seedling had burst.
The differences between the bud burst percentages of the intact and cut seedlings in transfers were analyzed using logistic regression with a binary response for the terminal buds and a binomial response for the four uppermost lateral buds of the seedlings. The differences between the average time to bud burst were analyzed using analyses of variance with a log transformed response variable. In all the models the experimental unit was a seedling and the explanatory variables were seedling type (intact/cut), transfer and their interaction. Statistical analyses were carried out using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
3 Results
In the first two transfers on 2 October and 16 October no bud burst was observed in the subsequent forcing conditions in either the intact seedlings or the cut seedlings (BB% ≈ 0%; Fig. 2). With successive later transfers with more accumulated chilling units (Fig. 1) the values of BB% increased towards 100 % according to S-shaped pattern, indicating further endodormancy release (Fig. 2).
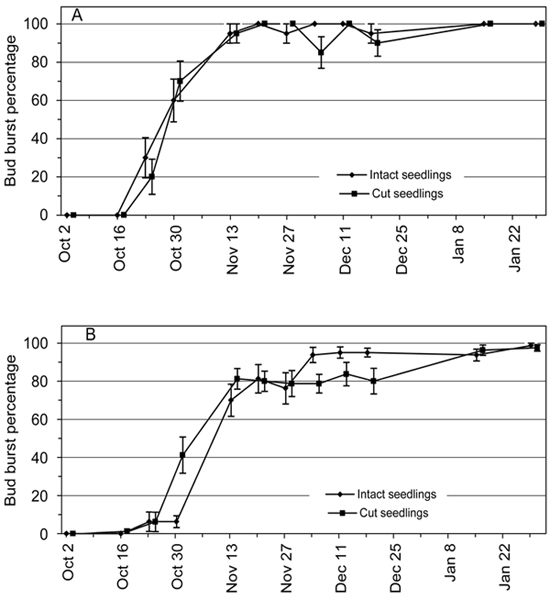
Fig. 2. Bud burst percentage (BB%) of 2-year-old Norway spruce seedlings in relation to the 12 transfer times from the natural and preforcing (transfers from 13 November onwards) conditions to the forcing conditions. BB% is shown separately for the intact seedlings and the seedlings where the root connection was cut by detaching the shoot from the root system. Bars = standard error of mean. (A) Terminal bud; (B) four uppermost lateral buds of the seedling.
There were no significant differences between the cut seedlings and the intact seedlings in the percentage of terminal buds which burst (p = 0.992) (Fig. 2A). The overall pattern of BB% in the four uppermost lateral buds per seedling was similar to the pattern of terminal buds but a statistically significant interaction of the seedling types and transfers (p < 0.001) was found (Fig. 2B). In the transfers on 30 October and 13 November the BB% of four uppermost lateral buds was slightly higher in the cut seedlings than in the intact seedlings but in transfers on 4, 11 and 18 December this effect was reversed so that the BB% values of the cut seedlings were lower than in intact seedlings (Fig. 2B). In other transfers the BB% values of the two experimental groups were about the same.
The average days to bud burst, DBB, decreased continuously from the early transfers to the late ones (Fig. 3), indicating further release of endodormancy with additional accumulation of chilling (Fig. 1). In addition, the DBB values of the cut seedlings were systematically higher than DBB values of the intact seedlings (Fig. 3). The average DBB difference of cut and intact seedlings was 3.4 days in the terminal buds and 4.6 days in the two out of four uppermost lateral buds. The DBB difference of terminal buds between the cut and intact seedlings was significant (p < 0.001) (Fig. 3A). The DBB difference of the two out of four uppermost lateral buds per seedling was significant (p < 0.001) but significant interaction of the seedling types and transfers (p = 0.018) was found (Fig. 3B). However, in spite of interaction the DBB values of cut seedlings were consistently higher than those of the intact seedlings regardless of the transfer (Fig. 3B).
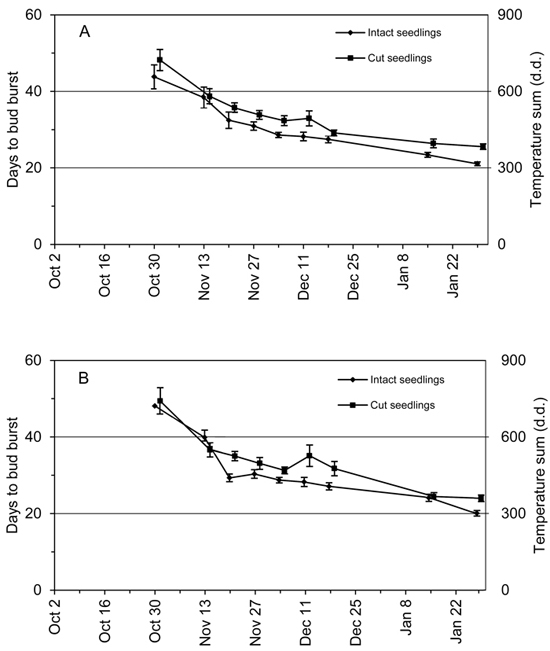
Fig. 3. Average days to bud burst (DBB) of 2-year-old Norway spruce seedlings in relation to the time of transfer from the natural and preforcing (transfers from 13 November onwards) conditions to the forcing conditions. DBB is shown separately for the intact seedlings and the seedlings where the root connection was cut by detaching the shoot from the root system. Bars = standard error of mean. (A) Terminal bud; (B) bud burst of at least two out of four uppermost lateral buds of the seedling. The vertical axis on the right indicates the approximate temperature sum in forcing conditions corresponding to the DBB.
4 Discussion
The increasing pattern of bud burst percentage, BB% (Fig. 2), is in accordance with earlier studies (e.g. Nienstaedt 1967; Hänninen 1990). The cutting of the root connection by detaching the shoots from the root system did not affect the dormancy release of terminal buds, as measured by bud burst percentage in the subsequent forcing conditions. BB% of the four observed lateral buds per seedling exceeded 80 % faster in the cut seedlings than in the intact seedlings. However, BB% of the cut seedlings exceeded 90 % much slower than in the intact ones.
The time required for vegetative bud burst in forcing conditions, DBB, was generally reduced as chilling accumulated during autumn and early winter both in the intact and cut seedlings. This inverse relationship of chilling and forcing on the timing of bud burst has been demonstrated in spruce species in earlier studies (e.g. Nienstaedt 1967; Worrall and Mergen 1967). Although the general pattern of DBB with chilling was the same for both treatments, detaching the root system delayed the timing of bud burst by 3.4 to 4.6 days.
Viherä-Aarnio et al. (2014) studied microscopically the internal development of buds in twigs of 17-year-old Norway spruce trees. After transfer on 20 November (chilling unit sum 846 ch.u.), the primordial shoot began rapid elongation at 150 d.d. (T ≥ +5 °C) (Viherä-Aarnio et al. 2014). In the present study the transfer on 20 November (chilling unit sum 810 ch.u.), was the earliest one in which the bud burst percentage of the terminal buds of the seedlings reached 100% indicating the achievement of ontogenetic competence (Hänninen and Kramer 2007) and transition from endodormancy to ecodormancy (Hänninen 1995; Junttila and Hänninen 2012). Thus, taken together, the observations of visible bud burst of seedlings in the present study and those addressing internal development in buds of adult trees (Viherä-Aarnio et al. 2014) both indicate a culmination of release of endodormancy in mid-November with chilling unit sum 800–850 ch.u.
In conclusion, in the present study cutting the root connection by detaching the shoot from the root system slightly delayed the date of bud burst in young Norway spruce seedlings, whereas no consistent effect on bud burst percentage was found. Our preliminary seedling data suggest that using detached tree material in dormancy release experiments may affect the results but it will evidently not lead to drastically erroneous conclusions. However, further studies, using different seed lots, are needed to assess the effect of detaching on the dormancy release and bud burst, especially in adult trees.
Acknowledgements
We thank Jouko Lehto for his assistance in the greenhouse experiment, Jaakko Heinonen for statistical advice, Pekka Voipio for figure preparation and two anonymous referees for their valuable comments on the manuscript. This study was financially supported by the Ella and Georg Ehrnrooth Foundation, the Niemi Foundation, the Finnish Cultural Foundation; South Savo Regional Fund and the the Foundation for Forest Tree Breeding, in the form of a research grant to Jouni Partanen.
References
Arora R., Rowland L.J., Tanino K. (2003). Induction and release of bud dormancy in woody perennials: a science comes of age. Horticultural Science 38: 911–921.
Basler D., Körner C. (2012). Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agricultural and Forest Meteorology 165: 73–81. http://dx.doi.org/10.1016/j.agrformet.2012.06.001.
Campbell R.K., Sugano A.I. (1975). Phenology of bud burst in Douglas-fir related to provenance, photoperiod, chilling and flushing temperature. Botanical Gazette 136: 290–298. http://dx.doi.org/10.1086/336817.
Chao W.S., Foley M.E., Horvath D.P., Anderson J.V. (2007). Signals regulating dormancy in vegetative buds. International Journal of Plant Developmental Biology 1: 49–56.
Cooke J.E., Eriksson M.E., Junttila O. (2012). The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant, Cell & Environment 35: 1707–1728. http://dx.doi.org/10.1111/j.1365-3040.2012.02552.x.
Coville F.V. (1920). The influence of cold in stimulating the growth of plants. Journal of Agricultural Research 20: 151–160.
Garner W.W., Allard H.A. (1923). Further studies in photoperiodism, the response of the plant to relative length of day and night. Journal of Agricultural Research 23: 871–920.
Hannerz M. (1994). Predicting the risk of frost occurrence after budburst of Norway spruce in Sweden. Silva Fennica 28: 243–249. http://dx.doi.org/10.14214/sf.a9175.
Hänninen H. (1990). Modelling bud dormancy release in trees from cool and temperate regions. Acta Forestalia Fennica 213: 1–47.
Hänninen H. (1995). Effects of climatic change on trees from cool and temperate regions: an ecophysiological approach to modelling of bud burst phenology. Canadian Journal of Botany 73: 183–199. http://dx.doi.org/10.1139/b95-022.
Hänninen H., Backman R. (1994). Rest break in Norway spruce seedlings: test of a dynamic temperature response hypothesis. Canadian Journal of Forest Research 24: 558–563. http://dx.doi.org/10.1139/x94-073.
Hänninen H., Kramer K. (2007). A framework for modelling the annual cycle of forest trees in boreal and temperature regions. Silva Fennica 41(1): 167–205. http://dx.doi.org/10.14214/sf.313.
Hänninen H., Pelkonen P. (1989). Dormancy release in Pinus sylvestris L. and Picea abies (L.) Karst. seedlings: effects of intermittent warm periods during chilling. Trees – Structure and Function 3: 179–184. http://dx.doi.org/10.1007/bf00226654.
Jablanczy A. (1971). Changes due to age in apical development in spruce and fir. Bi-monthly research notes – Canadian Forest Service 27: 10.
Junttila O., Hänninen H. (2012). The minimum temperature for budburst in Betula depends on the state of dormacy. Tree Physiology 32: 337–345. http://dx.doi.org/10.1093/treephys/tps010.
Lang G.A. (1994). Dormancy – the missing links – molecular studies and integration of regulatory plant and environmental interactions. Horticultural Science 29: 1255–1263.
Lang G.A., Early J.D., Martin G.C., Darnell R.L. (1987). Endo-, para- and ecodormancy: physiological terminology and classification for dormancy research. Horticultural Science 22: 371–377.
Nienstaedt H. (1966). Dormancy and dormancy release in white spruce. Forest Science 12: 374–384.
Nienstaedt H. (1967). Chilling requirements in seven Picea species. Silvae Genetica 16: 65–68.
Partanen J., Hänninen H., Häkkinen R. (2005). Bud burst in Norway spruce (Picea abies): preliminary evidence for age-specific rest patterns. Trees – Structure and Function 19: 66–72. http://dx.doi.org/10.1007/s00468-004-0364-5.
Repo T., Mela M., Valtanen J. (1984). Männyn versosyövälle alttiiden ja vastustuskykyisten taimialkuperien erottaminen neulasten ominaisuusimpedanssin mittauksella. [In Finnish with English Summary: Separation of susceptible and resistant provenances of Scots pine to Gremeniella abietina by specific needle impedance]. Folia Forestalia 610: 1–11.
Sarvas R. (1972). Investigations on the annual cycle of development of forest trees. Active period. Communicationes Instituti Forestalis Fenniae 76: 1–110.
Sarvas R. (1974). Investigations on the annual cycle of development of forest trees II. Autumn dormancy and winter dormancy. Communicationes Instituti Forestalis Fenniae 84: 1–101.
Søgaard G., Johnsen Ø., Nilsen J., Junttila O. (2008). Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiology 28: 311–320. http://dx.doi.org/10.1093/treephys/28.2.311.
Sutinen S., Partanen J., Viherä-Aarnio A., Häkkinen R. (2012). Development and growth of primordial shoots in Norway spruce before visible bud burst in relation to time and temperature in the field. Tree Physiology 32: 987–997. http://dx.doi.org/10.1093/treephys/tps063.
Viherä-Aarnio A., Sutinen S., Partanen J., Häkkinen R. (2014). Internal development of vegetative buds of Norway spruce trees in relation to accumulated chilling and forcing temperatures. Tree Physiology 34: 547–556. http://dx.doi.org/10.1093/treephys/tpu038.
Vitasse Y., Basler D. (2014). Is the use of cuttings a good proxy to explore phenological responses of temperate forests in warming and photoperiod experiments? Tree Physiology 34: 174–183. http://dx.doi.org/10.1093/treephys/tpt116.
Wareing P.F. (1956). Photoperiodism in woody plants. Annual Review of Plant Physiology 7: 191–214. http://dx.doi.org/10.1146/annurev.pp.07.060156.001203.
Wareing P.F., Saunders P.F. (1971). Hormones and dormancy. Annual Review of Plant Physiology 22: 261–288. http://dx.doi.org/10.1146/annurev.pp.22.060171.001401.
Worrall J., Mergen F. (1967). Environmental and genetic control of dormancy in Picea abies. Physiologia Plantarum 20: 733–745. http://dx.doi.org/10.1111/j.1399-3054.1967.tb07217.x.
Total of 30 references.

