Micropropagation of threatened black alder
San José M. C., Janeiro L. V., Corredoira E. (2013). Micropropagation of threatened black alder. Silva Fennica vol. 47 no. 1 article id 892. https://doi.org/10.14214/sf.892
Abstract
Micropropagation techniques are valuable tools for propagating, conserving and restoring trees. An efficient micropropagation method involving axillary shoot proliferation of material obtained from mature European alder (Alnus glutinosa (L.) Gaertn.) trees was developed. Branch segments from trees aged 20–30 years were forced to flush, and explants derived from new shoots were cultured on Woody Plant Medium supplemented with 8.88 µM benzyladenine and 2.85µM indole-3-acetic-acid. In vitro establishment was achieved in all five genotypes evaluated. Shoot cultures were maintained by sequential subculture of explants on the same medium supplemented with 0.88–0.44 µM benzyladenine and 2.85 µM indole-3-acetic acid. Transfer to fresh medium every 3 weeks during a 9-week multiplication period and the inclusion of 2.28 µM zeatin during the last 3 weeks of culture improved the multiplication rate and shoot quality. Use of 2% glucose as the carbohydrate source produced better results than 3% sucrose for shoot proliferation. In vitro rooting of shoots was achieved with 2% glucose and 0.49 µM indole-3-butyric acid for 7 days, followed by in vitro culture on auxin-free medium for 21 days. Rooted plantlets were acclimatized to the greenhouse and were viable for reintroduction into the natural habitat.
Keywords
Alnus glutinosa;
micropropagation;
glucose;
mature trees
-
San José,
Instituto de Investigaciones Agrobiológicas de Galicia (IIAG), CSIC, Apartado 122, 15080 Santiago de Compostela, Spain
E-mail
sanjose@iiag.csic.es

- Janeiro, INLUDES, Diputación Provincial de Lugo, Ronda de la Muralla 140, 27004 Lugo, Spain E-mail lauravj68@hotmail.com
- Corredoira, Instituto de Investigaciones Agrobiológicas de Galicia (IIAG), CSIC, Apartado 122, 15080 Santiago de Compostela, Spain E-mail elenac@iiag.csic.es
Received 18 September 2012 Accepted 21 December 2012 Published 11 June 2013
Views 135963
Available at https://doi.org/10.14214/sf.892 | Download PDF

1 Introduction
Alders are not often the focus of forest protection concerns in Europe in spite of the ecological value of the genus being well recognized (Cech 1998). European alder (Alnus glutinosa (L.) Gaertn.), also called black alder or European black alder, has considerable landscape value along waterways, plays a vital role in riparian ecosystems, and the root system stabilize riverbanks (Gibbs et al. 1999). Black alder populations have declined drastically throughout Europe in recent years, partly as a result of deforestation and the disappearance of characteristic riparian habitats, although mainly because of alder blight disease, caused by Phytophthora alni Brasier et al. and subspecies (Brasier et al. 2004).
The incidence of alder blight disease has increased steadily since it was first described in 1993 (Gibbs et al. 1994), and very high losses have occurred in some areas, such as southeast England, northeastern France, Bavaria and northern Spain (Gibbs 1995; Gibbs et al. 1999; Streito et al. 2002; Jung and Blaschke 2004; Tuset et al. 2006). Heavy loss of alders as a result of P. alni infection may have serious effects on forest and soil composition, wildlife food and habitats, and soil erosion (Cree 2006).
Conservation of valuable genotypes of black alder is therefore imperative. In this regard, efforts should focus on ex situ conservation, with germplasm collections of the most important populations of alders in different zones, as in situ conservation is very difficult given the advanced state of the disease. Although conservation of the alder germplasm is possible by cold stratification and cryopreservation of seeds (Pawel 2010), storage of this material is of little interest, because seeds are genetically heterogeneous. Vegetative propagation of A. glutinosa through cuttings is possible by use of standard horticultural techniques (Périnet and Lalonde 1983); however, micropropagation methods would be beneficial for the large-scale multiplication, improvement, and conservation of this species.
Axillary shoot proliferation from cultured meristems is the most frequently used method for micropropagation as this provides genetic stability and is easily attained in many plant species (George et al. 2008). In black alder, this in vitro culture technique would provide short-to-medium term ex situ storage of valuable genotypes and massive production of the clonal plant stock to restore areas devastated by the disease. In addition, micropropagation protocols are a prerequisite for cryopreservation and genetic transformation methods, which can complement conventional breeding programs. Efficient methods are required for the clonal propagation of A. glutinosa that have been selected at a mature stage, because it is difficult to predict the characteristics of a mature tree from plants still in the juvenile phase and moreover, most individuals that require protection from the disease are already adult trees. Micropropagation by axillary shoot multiplication has been achieved in several species of genus Alnus although most of these reports refer to material of juvenile origin, such as seedlings or young trees (Garton et al. 1981; Périnet and Lalonde 1983; Tremblay and Lalonde 1984; Tremblay et al. 1986; Périnet and Tremblay 1987; Barghchi 1988). Despite technological advances that have been made over the years, there have been few reports regarding of the propagation of mature Alnus trees (Périnet et al. 1988; Lall et al. 2005; Gailite and Auzenbaha 2010).
In view of the limited success of previous approaches to the in vitro culture of mature material from A. glutinosa, and the interest in defining the optimal conditions for clonal micropropagation and conservation, the main objectives of this research were: 1) to study the initiation and stabilization stages of shoot cultures derived from adult trees of A. glutinosa; 2) to optimize the shoot proliferation stage by evaluating the effect of different cytokinins and carbon source treatments; 3) to define the rooting ability of micropropagated shoots for plantlet regeneration and subsequent acclimatization of this species.
2 Material and methods
2.1 Establishment
The plant material was collected in June and November 2009, and in November 2010. The source material consisted of crown branches from A. glutinosa trees (R1 to R4), aged 25–30 years, growing in the “Terras do Miño” Biosphere Reserve in the province of Lugo (NW Spain) (43°0´N, 7°33´W), and basal sprouts obtained from the base of a tree (G1), aged between 20–25 years, from the surroundings of Santiago de Compostela (NW Spain) (42°52´N, 8°32´W). The branches were cut in 20–25 cm segments ranging from 0.5–3 cm in diameter, placed upright in moistened perlite, and forced to flush axillary shoots in a growth chamber at 25 °C and 80–90% relative humidity, under a 16 h photoperiod (90–100 µmol m–2 s–1 provided by cool-white fluorescent lamps). Twenty to 40 branch segments were used for each collection date and genotype, and after 3–4 weeks the proportion of branch segments producing new sprouted shoots was recorded. Flushed shoots 3–10 cm in length were stripped of leaves, rinsed under tap water for 5–10 min, and surface disinfected by successive immersion in 70% ethanol for 20 s, 0.6% solution of free chlorine (Millipore® chlorine tablets) containing 2–3 drops of the desurfactant Tween 80® for 5 min, and then rinsed three times in sterile distilled water. Explants consisting of 5–8 mm shoot tips and nodal segments bearing 1 or 2 axillary buds were excised from the flushed shoots, and placed upright in 30 × 150 mm culture tubes containing 20 ml of Woody Plant Medium (WPM, Lloyd and McCown 1980) supplemented with 30 g l–1 sucrose, 7 g l–1 agar, 8.88 µM benzyladenine (BA) and 2.85 µM indole-3-acetic acid (IAA) (basal medium).
To avoid excreted substances, each explant was moved to the opposite side of the tube one day after initiation of culture, and the explants were then transferred to fresh medium every two weeks until the cultures stabilized. All cultures were maintained in a growth chamber with a 16 h photoperiod (50–60 µmol m–2 s–1 provided by cool-white fluorescent lamps) and temperatures of 25 °C (light) and 20 °C (dark). These standard culture conditions were also applied to the shoot multiplication and rooting stages.
2.2 Shoot multiplication
New shoots longer than 1 cm were excised from the original explants and transferred to tubes containing basal medium supplemented with 0.88 µM BA and 2.85 µM IAA and were subcultured every 4–5 weeks in the same medium. To improve shoot development, the shoots were transferred every three weeks to fresh medium during a multiplication period of 9 weeks. Basal medium containing 0.88 µM BA and 2.85 µM IAA was used for the first 3 weeks, and the concentration of BA was then halved for the next 6 weeks. Since in vitro establishment of the G1 and R4 material was easily achieved and a large number of shoots were obtained from these materials, cultures derived from theses genotypes were used to investigate shoot proliferation. In all multiplication experiments, initial explants consisted of 8–10 mm shoot tips and basal stem segments bearing 1–2 nodes.
The influence of zeatin on shoot multiplication was evaluated on the basis of preliminary studies with Fagus spp. and Quercus spp. (Vieitez et al. 2003, 2009). Shoot explants from genotype R4 were cultured on basal medium with two proliferation regimes:
- Regime 1: Cycle 1: 0.88 µM BA and 2.85 µM IAA; Cycle 2: 0.44 µM BA and 2.85 µM IAA; Cycle 3: 0.44 µM BA, 2.85 µM IAA and 2.28 µM zeatin. Each subculture cycle lasted 3 weeks.
- Regime 2: The same as regime 1, but with 2.28 µM zeatin in all cycles.
Control without zeatin was included.
To optimize shoot production and quality of A. glutinosa cultures, the influence of type and concentration of carbon source was studied by culturing shoots of genotypes G1 and R4 on basal medium supplemented with 111 or 166 mM glucose or 58 or 87 mM sucrose (2 or 3%, respectively). Regime 1 was used in this experiment.
In both experiments the following variables were determined at the end of the 9-week multiplication period: response percentage defined as the percentage of explants forming shoots; the mean number of 0.5–1 cm long shoots; the mean number of shoots longer than 1 cm, and the mean length of the longest shoots (mm). Eighteen explants were used per genotype and treatment, and both experiments were repeated three times. In total, 324 explants were used in the first experiment, and 432 in the second.
2.3 Rooting
Rooting experiments were performed with G1 and R4 genotypes. Shoots 1.5 to 3 cm in length were isolated from multiplication cultures and placed for 7 days in root induction medium consisting of WPM with half-strength macronutrients, 111 mM glucose (2%) or 87 mM sucrose (3%), 7 g l–1 Difco agar, and supplemented with 0or 0.49 µM indole-3-butyric acid (IBA). The shoots were then transferred to fresh medium of the same composition without IBA (root expression medium) for the remainder of the 1-month rooting period. The experiment was set as a 2 × 2 factorial design based on carbohydrate source with or without IBA. Twelve shoots were evaluated per genotype and treatment and the experiments were repeated three times. In total, 288 shoots were used. At the end of the 1-month rooting period, the following variables were determined: percentage of rooted shoots, mean number of roots per shoot, and the length of the longest root of each shoot (mm).
2.4 Acclimatization
Rooted plantlets of G1 and R4 genotypes were rinsed free of agar and transferred to pots (300 ml, 180 × 60 mm) containing a mixture of commercial substrate (Pinot ®, Siffly, The Netherlands) and perlite (3:1 v/v), or normal garden soil. Potted plantlets were placed in a growth chamber at a 90% relative humidity, 25 ± 2 °C, with a 16 h photoperiod (90–100 µmol m–2 s–1 provided by cool-white fluorescent lamps) for 3 weeks, and then transferred to a greenhouse under conditions of natural daylight. Plants were fertilized weekly with 10 ml of Hoagland’s solution (Hoagland and Arnon 1941). Twenty plants per genotype were used for each type of substrate, and the experiment was repeated twice. In total, 160 plants were used. The survival rates in the greenhouse were recorded after 6 weeks.
2.5 Statistical analysis
Data were expressed as means ± standard errors (SE). The influence of the experimental factors was analysed by Student’s test (MedCalc version 10.3, Marekarke, Belgium) for the data shown in Table 2. The data showed in Table 3 and 4 were analysed by one-way analysis of variance (ANOVA I) and two-way analysis of variance (ANOVA II). Differences were considered significant at p ≤ 0.05. An arcsine square root transformation was applied to normalize percentage values data prior analysis. Non-transformed data are shown in the tables and figures.
3 Results
3.1 Establishment
Flushing occurred in branch segments of all genotypes evaluated after 10–15 days in the growth chamber (Fig. 1A). The percentage of shoot development was affected by the collection date and the genotype (Table 1). The best results were obtained with genotype G1, which displayed more juvenile characteristics than genotypes R1 to R4 obtained from crown material. The thickness of the stem cuttings also affected the percentage of shoot development, and the best results were obtained with 0.5–2 cm thick cuttings. In those genotypes (R1, R3 and R4) from which material was collected on two occasions, in June and November, the best results were obtained in November. The rates of contamination were also affected by the time of year that the material was collected, and were higher in November. Explant survival was higher than 50% for all genotypes evaluated, and apical explants were generally more reactive than nodal explants, 60–79% and 30–40% respectively. Necrosis and callus formation were more evident in the latter; the calluses grew from the cut surfaces and tended to enclose the entire explants. Transfer of the initial explants to another area of the culture medium within the same tube after 24 h reduced the negative effects of phenolic compounds and other exudates. After 6–8 weeks in culture, new shoots were observed in the five genotypes studied. Genotypes differed in the success of the in vitro establishment, and shoot formation was most successful in G1 and R4.
3.2 Shoot multiplication
Shoot development was poor and many explants were lost because of a progressive decline in vigor, when the shoots were subcultured every 4–5 weeks in basal medium with 0.88 µM BA plus 2.85 µM IAA. The response percentages (from 60% to 93%), the number (from 1.1 to 2.0) and length of the shoots (from 10 to 15 mm) increased greatly when shoots were transferred to fresh medium every 3 weeks in a 9-week multiplication period. Frequent transfer to fresh medium was important to prevent apical senescence, to increase multiplication rates and to reinvigorate the cultures. All black alder genotypes became stabilized during this procedure, although the time required ranged from 5 months (genotype G1) to 10–12 months (genotypes R1 to R4) (Table 1). In all cases, it was possible to reculture subcultured tissue repeatedly on fresh medium, after successive harvesting of the most vigorous shoots. However, in genotypes R1 to R4 short shoots with reduced leaf development were observed. To address these problems, further experiments were performed.
| Table 1. Effect of genotype and collection date on the in vitro establishment of shoot cultures of several adult clones of Alnus glutinosa. | ||||||||
| Clone | Flushing capacity (%) | Number of initial explants | Contamination rate (%) | Period needed for stabilization (months) | ||||
| June | November | June | November | June | November | June | November | |
| R1 | 15.0 | 66.7 | 22.0 | 25.0 | 9.1 | 56.0 | 12 | 12 |
| R2 | 44.4 | - | 24.0 | - | 25.0 | - | 10 | - |
| R3 | 33.3 | 77.8 | 20.0 | 21.0 | 10.0 | 57.1 | Lost | 10 |
| R4 | 43.8 | 60.0 | 21.0 | 20.0 | 23.8 | 50.0 | Lost | 10 |
| G1 | 94.1 | - | 40.0 | - | 15.0 | - | 5 | - |
Inclusion of zeatin in the multiplication medium improved shoot development and leaf expansion (Table 2). The percentage responses in all regimes evaluated were high, close to 100%. The presence of zeatin produced a significant increase in the total number of shoots, obtaining the best results with regime 1. The presence of zeatin during the entire multiplication period (regime 2) improved shoot elongation and resulted in a significant increase in the number of shoot longer than 1 cm (p ≤ 0.05). In view of the results, regime 1 (more economical) was selected for proliferation of all alder genotypes.
| Table 2. Effect of zeatin regime on the in vitro multiplication of Alnus glutinosa (genotype R4). Data were collected at the end of a 9-week multiplication period. | |||||
| Treatment | Response (%) | No. of shoots 0.5–1.0 cm 2) | No. of shoots ≥ 1.0 cm 2) | Total no. of shoots 2) | Longest shoot length (mm) 2) |
| Control | 92.6±1.83 | 1.0±0.41a | 0.9±0.09a | 2.0±0.35a | 14.9±0.38a |
| Regime 1 1) | 96.3±3.70 | 2.1±0.17b | 1.0±0.11a | 3.1±0.15b | 15.0±0.79a |
| Regime 2 | 100.0±0.00 | 1.5±0.18a | 1.3±0.16b | 2.8±0.21c | 16.5±0.79b |
| 1) Regime 1: Cycle 1: 0.88 µM BA + 2.85 µM IAA, cycle 2: 0.44 µM BA + 2.85 µM IAA, cycle 3: 0.44 µM BA + 2.85 µM IAA + 2.28 µM zeatin. Regime 2: as regime 1 with 2.28 µM zeatin in all cycles. Control without zeatin. Values represent means ± SE for three replications. 2) In each column, values followed by the same letter are not significant at p ≤ 0.05 level using Student’s test | |||||
In a subsequent experiment, the influence of the carbohydrate source added to the culture medium was studied in shoots of genotypes G1 and R4 (Fig. 1B, Table 3). In both genotypes, the total number of shoots, number of shoots longer than 1 cm, and shoot length were significantly higher (p ≤ 0.05) when glucose was used as the carbohydrate source. The highest concentration of glucose (3%) produced the highest proliferation rates, i.e. total number of shoots, although some signs of hyperhydricity appeared at this concentration, with thicker stems and leaves. The results indicate that the type of carbohydrate had a greater influence than the concentration. On the basis of the results with genotype G1, another experiment was carried out with genotype R4, to compare the results with 2% glucose and 3% sucrose. As occurred with G1, the best multiplication rates were obtained by addition of 2% glucose to the culture medium, with significant differences in all of the variables studied (p ≤ 0.05), except the number of shoots 0.5–1 cm and the response percentages which were very close to 100% in both cases.
| Table 3. Effect of carbohydrate source (S: sucrose, G: glucose) on the in vitro multiplication of Alnus glutinosa (Genotypes G1 and R4). Data were collected after 9-week multiplication period. | ||||||||||||||
| Sugar (%) | Response (%) 1) | No of shoots 0.5–1 cm | No of shoots ≥ 1 cm 2) | Total no of shoots 2) | Shoot length (mm) 2) | |||||||||
| S | G | S | G | S | G | S | G | S | G | |||||
| G1 | ||||||||||||||
| 2% | 92.4±0.9 | 100±0.0 | 0.5±0.02 | 0.8±0.02 | 1.1±0.02 | 2.2±0.08 | 1.7±0.02 | 3.1±0.08 | 16.8±0.11 | 23.0±0.44 | ||||
| 3% | 100±0.0 | 100±0.0 | 0.7±0.03 | 1.1±0.07 | 1.2±0.01 | 2.3±0.08 | 1.9±0.02 | 3.5±0.13 | 17.2±0.37 | 22.4±0.33 | ||||
| Mean | 1.2±0.06a | 2.3±0.03b | 1.8±0.1a | 3.3±0.2b | 17.0±0.2a | 22.7±0.3b | ||||||||
| R4 | ||||||||||||||
| G 2% | 98.0±0.5 | 1.4±0.11 | 2.4±0.04a | 3.8±0.08a | 18.0±0.11a | |||||||||
| S 3% | 98.1±0.4 | 0.7±0.03 | 1.4±0.05b | 2.1±0.08b | 16.3±0.20b | |||||||||
| 1) Percentage of explants that forming shoots. Values represent means ± SE for three replications. 2) In each column, values followed by the same letter are not significantly different at the P ≤ 0.05 level, according to the Least Significant Difference (LSD) test. | ||||||||||||||
In view of the results obtained, the protocol established for multiplication of alder genotypes included regime 1 subculture and the addition of 2% glucose as the source of carbohydrate.

Fig. 1. Micropropagation by axillary shoot proliferation of Alnus glutinosa. A. Forced flushing of branch sections in the growth chamber. B. Shoot multiplication in genotype G1 after culture in WPM medium (Regime 1) supplemented with glucose 2%. C. Root development on shoots of genotype R4 treated with 0.49 µM IBA and glucose 2%. D. Acclimatized plants (genotype R4) 6 weeks old.
3.3 Rooting
The rooting success of shoots of genotypes G1 and R4 are shown in Table 4. Root development was close to 80% in all treatments and followed a similar trend. Although there were no significant differences in the rooting percentages, the highest values were obtained when glucose was incorporated in the medium as the source of carbohydrate. The presence of auxin (0.49 µM IBA) in the glucose-containing medium increased the rooting success in both genotypes, although the increases were not significant. Unlike the percentage rooting, the number of roots was greater in the treatments with sucrose and auxin, with significant differences (p ≤ 0.05) in both genotypes. The interaction between carbohydrate source and auxin had significantly different effects (p ≤ 0.01) on the number of roots for genotype G1. The effect of the sugars on the root length was different in each genotype, in G1 the best results were obtained with glucose (p ≤ 0.01), whereas in R4 the best results were obtained with sucrose, although in this case the differences were not significant. The presence of auxin in the rooting medium increased the speed of root formation, independently of the carbohydrate used (Fig. 2). In genotype G1, root emergence was initiated after day eight in all treatments; when auxin was included in the medium, rooting concluded on day 14–16, whereas in medium without auxin, completion of rooting was delayed until day 20–22. A similar trend was observed with genotype R4, although the rooting percentage was lower. Roots had a similar appearance to those developed ex vitro, with the presence of secondary roots, a good connection to the shoots, and no callus was observed at the base of the shoots (Fig. 1C).
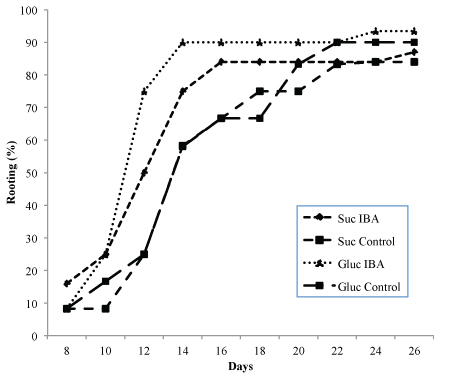
Fig. 2. Rooting ability of Alnus glutinosa shoots (Genotype G1) relative to incubation time. Shoots were incubated in rooting medium with or without 0.49 µM IBA and glucose 2% or sucrose 3% as carbohydrate source.
| Table 4. Effect of carbohydrate source and auxin (0.49 µM IBA) on root formation in shoots from Alnus glutinosa (Genotypes G1 and R4). Data were collected after 4 weeks. | ||||||
| Rooting treatment | Rooting (%) | Number of roots 1) | Length of longest root (mm) 1) | |||
| Sucrose 3% | Glucose 2% | Sucrose 3% | Glucose 2% | Sucrose 3% | Glucose 2% | |
| G1 | ||||||
| –IBA | 84.0±8.0 | 90.0±10.0 | 2.1±0.1a | 2.1±0.1a | 28.2±0.5 | 30.3±4.5 |
| +IBA | 86.9±2.5 | 93.4±6.7 | 2.3±0.1b | 2.0±0.1a | 29.5±1.4 | 32.3±0.3 |
| Mean | 28.9±0.6a | 31.3±1.0b | ||||
| R4 | ||||||
| –IBA | 79.0±2.4 | 79.3±0.7 | 2.5±0.2 | 2.1±0.2 | 32.4±5.0 | 29.7±0.6 |
| +IBA | 79.4±2.4 | 85.7±1.1 | 2.6±0.4 | 2.1±0.1 | 34.1±5.0 | 29.2±1.4 |
| Mean | 2.3±0.0a | 2.1±0.0b | ||||
| Values represent means ± SE for three replicates. 1) In each column, values followed by the same letter are not significantly different at the P ≤ 0.05 level, according to the Least Significant Difference (LSD) test. | ||||||
3.4 Acclimatization
After 1 month in the rooting medium, shoots that had formed roots were successfully acclimatized in a growth chamber. New growth had been initiated after 3 weeks and surviving plantlets were transferred to the greenhouse. There were no significant differences in the acclimatization response with the different substrates. After 6 weeks in the greenhouse the survival rates ranged between 90–95% (Pinot® and perlite and garden soil, respectively) for both genotypes. Plants continued to grow vigorously in the greenhouse (Fig. 1D).
4 Discussion
This study describes the protocols for shoot establishment, proliferation and plant regeneration of adult black alder. Juvenile explants are often used in micropropagation because explants from mature trees are more difficult to propagate in vitro. However, Bonga et al. (2010) reported that in hardwood trees and a few gymnosperms, responsive tissues can be found in the root-shoot junction, in root or stump sprouts, sphaeroblasts, and epicormic shoots, making in vitro propagation easier. The present results showed that basal shoots from adult tree (G1) were more responsive than crown material (R1 to R4) for culture establishment. The use of a growth chamber to flush shoots of black alder crown branch segments enabled us to obtain vigorous explants. It appears that this material was sufficiently reinvigorated to provide reactive explants. Rejuvenation by forced flushing of branch segments has been described for several species (Vieitez et al. 1994; Gomes and Canhoto 2009; Vieitez et al. 2009). The results showed that for culture initiation, apical explants were more reactive than nodal explants. Similar results have been reported for other woody species such as Eucalyptus nitens Deane &. Maiden (Gomes and Canhoto 2003) and Arbutus unedo L. (Gomes and Canhoto 2009). In the latter study, the authors suggested higher production of exudates from nodal explants or the presence in apical explants of leaf primordia which produce plant growth regulators that promote cell division and retard senescence as the possible causes of this behaviour.
In alder cultures, good multiplication of shoots in the presence of BA has been reported for cultures initiated from juvenile shoots of A. glutinosa (Garton et al. 1981; Périnet and Lalonde 1983; Tremblay and Lalonde 1984). However, the use of adult material presents more problems. Lall et al. (2005) established a protocol for multiplication of adult material of black alder which required the incorporation of 2,3,5-triiodobenzoic acid (TIBA), an auxin transport inhibitor. The hyperhydricity caused by TIBA was reversed after several subculture cycles without TIBA. Difficulties in the use of adult material may be related to the changes in growth characteristics that often accompany the transition from juvenile to mature stages in woody plants (Poething 1990). On the basis of previous studies carried out in different species of genera Fagus and Quercus (Vieitez et al. 2003, 2009), we decided to divide the multiplication period into three cycles each of 3 weeks, which improved the quality of the shoots obtained and the rates of multiplication. In Fagus sylvatica L., Vieitez et al. (1993) reported that transfer to fresh medium every 2 weeks could supply sufficient nutrients, thus favouring the in vitro development of shoots. Incorporation of zeatin in the culture medium improved the multiplication capacity of the shoots, thus favouring shoot elongation and leaf development. The in vitro growth of shoots and leaves of beech and oak species was also favoured by combining BA with zeatin (Vieitez et al. 2003, 2009). Zeatin is a natural cytokinin found in higher plants, and these authors suggested the occurrence of a possible imbalance in levels of endogenous zeatin or its various derivatives. This finding is consistent with those of Werner et al. (2001), who concluded that the growth of leaves in cytokinin-deficient tobacco plants not only requires cytokinins, but also fine adjustment of natural cytokinins levels.
Sugars, especially glucose, have been shown to regulate the expression of components of phytohormone-response pathways (Gibson 2004). In alders, the sugar requirements varied between species, as well as between genotypes at the intraspecific level, and played a particularly important role in the development of alder tissue cultures during the multiplication stage (Tremblay et al. 1986). Tremblay and Lalonde (1984) found that sucrose was optimum for A. glutinosa, whereas all other Alnus species grew better in glucose. The results of the present study showed that the performance of shoot cultures of mature A. glutinosa was affected by the type of sugar in the culture medium, and that glucose was better than sucrose for inducing shoot development. In many woody species, glucose has been reported to be quantitatively and qualitatively better than sucrose for shoot development (Harada and Murai 1996; Cuenca and Vieitez 2000; Vieitez et al. 2007).
Tremblay et al. (1986) reported the ease of rooting of shoots from juvenile material of different species of Alnus, often with 100% success in the presence or absence of auxin. In the present study, shoots established from mature material rooted easily, even in the treatment without auxin, although the presence of IBA in the medium increased the rooting percentages, especially when 2% glucose was incorporated into the medium. Périnet and Lalonde (1983) reported that in A. glutinosa, the presence of IBA in the rooting medium enhanced the number of roots per shoot and reduced the time necessary for 100% rooting. According to Corrêa et al. (2005), the presence of glucose in this phase may cause more cells to be recruited for root induction, thus improving the rooting response. Glucose exerts growth hormone-like activities and is one of the signaling molecules reported for gene expression, cell proliferation, root and inflorescence growth, and leaf expansion and senescence (Yanaglsawa et al. 2003; Cho et al. 2006). Independently of the treatments used, rooting of A. glutinosa was readily induced in vitro, thus permitting rapid multiplication of alders on a large scale.
The present study reports a simple and efficient protocol for the rapid propagation of Alnus glutinosa from branches of adult trees. Similar work has been done with juvenile material, and limited success has been achieved in culturing mature black alder material. However, this study highlights the importance of different culture protocols, in relation to the use of plant growth regulators and of glucose rather than sucrose as a key carbon source at proliferation and rooting stages, for successful proliferation of healthy shoots and plantlet regeneration. The protocol provides a successful and rapid method for the commercial propagation and ex situ conservation of mature black alder.
Acknowledgments
The authors thank José Carlos Suárez for excellent assistance in laboratory work. We also gratefully acknowledge Dr. A.M. Vieitez for useful advice and suggestions. This study was financed by INLUDES (Diputación Provincial de Lugo) through an agreement between CSIC-INLUDES.
References
Barghchi M. (1988). Micropropagation of Alnus cordata (Loisel.) Loisel. Plant Cell Tissue and Organ Culture 15(3): 233–244.
Bonga J.M., Klimaszewska K.K., von Aderkas P. (2010). Recalcitrance in clonal propagation, in particular in conifers. Plant Cell Tissue and Organ Culture 100(3): 241–254.
Brasier C.M., Kirk S.A., Delcan J., Cooke D.L., Jung T., Man in`t Veld M. (2004). Phytophthora alni sp nova and its variants: designation of a group of emerging heteroploid hybrid pathogens. Mycological Research 108(10): 1172–1184.
Cech T.L. (1998). Phytophthora decline of alder (Alnus spp.) in Europe. Journal of Arboriculture 24(6): 339–343.
Cho Y.H., Yoo S.D., Sheen J. (2006). Regulatory functions of nuclear hexokinase complex in glucose signaling. Cell 127(3): 579–589.
Corrêa L.R., Paim D.C., Schwambach J., Fett-Neto A.G. (2005). Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation 45(1): 63–73.
Cree L. (2006). Phytophthora alni. EXFOR Database. Available at: http://spfnic.fs.fed.us/exfor/data/pestreports.cfm
Cuenca B., Vieitez A.M. (2000). Influence of carbon source on shoot multiplication and adventitious bud regeneration in in vitro beech cultures. Plant Growth Regulation 32(1): 1–16.
Gailite A., Auzenbaha D. (2010). In vitro propagation of hybrid alder. Mezzinatne-Forest Science 21(54): 65–75.
Garton S., Hosier M.A., Read P.E., Farnham R.S. (1981). In vitro propagation of Alnus glutinosa Gaertn. Horticultural Science 16(3): 758–759.
George E.F., Hall M.A., De Klerk G.J. (2008). Micropropagation: uses and methods. In: George E.F., Hall M.A., De Klerk G.J. (eds.). Plant propagation by tissue culture. Exegetics, Basingstone, UK. p. 29–64.
Gibbs J.N. (1995). Phytophthora root disease of alder in Britain. OEPP/EPPO Bulletin. 25: 661–664.
Gibbs J.N., Lipscombe M.A., Peace A.J. (1999). The impact of Phytophthora disease on riparian populations of common alder (Alnus glutinosa) in southern Britain. European Journal of Forest Pathology 29(1): 39–50.
Gibbs J.N., Strouts R., Rose J., Brasier C. (1994). An usual Phytophthora associated with disease of common alder. Report on Forest Research. HMSO, London. p. 27–28.
Gibson S.I. (2004). Sugar and phytohormone response pathways: navigating a signaling network. Journal of Experimental Botany 55(395): 253–264.
Gomes F., Canhoto J.M. (2003). Micropropagation of Eucalyptus nitens Maiden (Shining gum). In Vitro Cellular & Developmental Biology-Plant 39(3): 316–321.
Gomes F., Canhoto J.M. (2009). Micropropagation of strawberry tree (Arbutus unedo L.) from adult plants. In Vitro Cellular & Developmental Biology-Plant 45(1): 72–82.
Harada H., Murai Y. (1996). Micropropagation of Prunus mume. Plant Cell Tissue and Organ Culture 46(3): 265–267.
Hoagland D.R., Arnon D.I. (1941). The water culture method for growing plants without soil. Miscellaneous Publications 3515. Circular of the California Agricultural Experimental Station 347–361.
Jung T., Blaschke M. (2004). Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread, and possible management strategies. Plant Pathology 53(2): 197–208.
Lall S., Mandegaran Z., Roberts A.V. (2005). Shoot multiplication in cultures of mature Alnus glutinosa. Plant Cell Tissue and Organ Culture 83(3): 347–350.
Lloyd G., McCown B.H. (1980). Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proceedings of the International Plant Propagator’s Society 30: 421–427.
Pawel, Ch. (2010). Cryopreservation of orthodox seeds of Alnus glutinosa. CryoLetters 31(2): 139–146.
Périnet P., Lalonde M. (1983). In vitro propagation and nodulation of the actinorhizal host plant Alnus glutinosa (L.) Gaertn. Plant Science Letters 29(1): 9–17.
Périnet P., Tremblay F.M. (1987). Commercial micropropagation of five Alnus species. New Forests 3(3): 225–230.
Périnet P., Vallaée G., Tremblay F.M. (1988). In vitro propagation of mature trees of Alnus incana (L.) Moench. Plant Cell Tissue and Organ Culture 15(1): 85–89.
Poething R.S. (1990). Phase change and the regulation of shoot morphogenesis in plants. Science 250(4983): 923–930.
Streito J-C., Legrand P.H., Tabary F., Jarnouen de Villartay G. (2002). Phytophthora disease of alder (Alnus glutinosa) in France: investigations between 1995 and 1999. Forest Pathology 32(3): 179–191.
Tremblay F.M., Lalonde M. (1984). Requirements for the in vitro propagation of seven nitrogen-fixing Alnus species. Plant Cell Tissue and Organ Culture 3(2): 189–199.
Tremblay F.M., Périnet P., Lalonde M. (1986). Tissue culture of Alnus spp. with regard to symbioses. In: Bajaj Y.P.S. (ed.). Biotechnology in agriculture and forestry, vol 1, trees 1. Springer-Verlag, Berlin, Heidelberg. p. 87–100.
Tuset J.J., González V., Hinarejos C., Mira J.L., Sánchez G. (2006). Prospección para determinar la posible presencia de Phytophthora spp. en las alisedas del norte de España. In: Cobos J.M. (ed.). Proceedings of the XXIII Annual Meeting of the Forest Health Working Group. Madrid, Spain. p. 11–15.
Vieitez A.M., Corredoira E., Ballester A., Muñoz F., Durán J., Ibarra M. (2009). In vitro regeneration of the important North American oak species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tissue and Organ Culture 98(2): 135–145.
Vieitez A.M., Ferro E., Ballester A. (1993). Micropropagation of Fagus sylvatica L. In Vitro Cellular & Developmental Biology-Plant 29(2): 183–188.
Vieitez A.M., San José M.C., Sánchez M.C., Ballester A. (2003). Micropropagation of Fagus spp. In: Jain S.M., Ishii K. (eds.). Micropropagation of woody trees and fruits. Kluwer Academic Publishers, The Netherlands. p. 181–215.
Vieitez A.M., Sánchez M.C., Amo-Marco J.B., Ballester A. (1994). Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropagation. Plant Cell Tissue and Organ Culture 37(3): 287–295.
Vieitez A.M., Sánchez M.C., García-Nimo M.L., Ballester A. (2007). Protocol for micropropagation of Castanea sativa Mill. In: Jain S.M., Häggman H. (eds). Protocols for micropropagation of woody trees and fruits. Springer, Heidelberg. p. 299–312.
Werner T., Motyka V., Strnada M., Schmülling T. (2001). Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences 98(18): 10487–10492.
Yanaglsawa S., Yoo S.D., Sheen J. (2003). Differential regulation of EIN3 stability by glucose and ethylene signaling in plantas. Nature 425(6957): 521–525.
Total of 38 references

