Timing and duration of short-day treatment influence morphology and second bud flush in Picea abies seedlings
Fløistad I. S., Granhus A. (2013). Timing and duration of short-day treatment influence morphology and second bud flush in Picea abies seedlings. Silva Fennica vol. 47 no. 3 article id 1009. https://doi.org/10.14214/sf.1009
Highlights
- The duration of short-day treatment, calculated as number of days, influenced the root collar diameter growth more than the timing of the treatment
- If short-day treatment starts early in summer, a longer duration of the treatment is recommended to avoid second bud flush.
Abstract
A slower reaction of diameter growth cessation compared to that of height growth in response to short day (SD) treatment is well documented in Picea abies (L.) Karst. seedlings, suggesting that the height/diameter ratio of seedlings could be controlled through appropriate timing and/or duration of SD treatment is forest nurseries. Here, we applied specific combinations of timing (starting date 20 and 27 June, 4 or 11 July) and duration (7, 10, 14 or 17 days) of SD treatment to assess the possibility of obtaining more sturdy seedlings. We observed a rapid and uniform height growth cessation following SD treatment compared with the delayed cessation of diameter growth. Height growth responded significantly only to starting date of SD treatment, resulting in taller seedlings for later starting dates. Diameter growth responded to the duration of SD treatment, with significantly less diameter growth in seedlings exposed to 14 or 17 days of SD treatment than in seedlings exposed to 7 or 10 days of SD treatment. Also starting date influenced diameter growth, resulting in significantly more diameter growth with the earliest starting date compared with the two latest starting dates of the SD treatment. A second bud flush occurred only in seedlings exposed to SD treatment starting on 20 or 27 of June and only following 7-14 days of duration. This implies a need of longer duration if the SD treatment starts early. This will be at the expense of sustained diameter growth, thus compromising the objective of obtaining more sturdy seedlings.
Keywords
Norway spruce;
photoperiod;
autumn bud break;
root collar diameter;
second bud break;
sturdyness
-
Fløistad,
Norwegian Institute for Agricultural and Environmental Research, Høgskolevn 7, N-1430 Ås, Norway & Norwegian Forest and Landscape Institute, P.O. Box 115, N-1431 Ås, Norway
E-mail
isf@skogoglandskap.no

- Granhus, Norwegian Forest and Landscape Institute, P.O. Box 115, N-1431 Ås, Norway E-mail aksel.granhus@skogoglandskap.no
Received 21 March 2013 Accepted 18 September 2013 Published 7 October 2013
Views 111808
Available at https://doi.org/10.14214/sf.1009 | Download PDF

1 Introduction
Artificial shortening of photoperiod by short-day (SD) treatment in late summer is a common measure in forest nurseries to induce growth cessation and frost hardiness development (Dormling et al. 1968; Heide 1974a; Colombo et al. 2001). Prior to autumn planting of Norway spruce (Picea abies (L.) Karst.) seedlings, 2–3 weeks of SD treatment is a common routine in forest nurseries in Norway, but SD treatment will also increase the hardiness level in seedlings intended for winter storage (L’Hirondelle et al. 2006). In addition, shoot elongation shows a more rapid response to the treatment than does diameter growth (Dormling et al. 1968; Heide 1974a; Bjørnseth 1977). SD treatment could therefore be used to control the height/diameter ratio to achieve more sturdy seedlings (Thompson 1985). This has especially been the case in years with a particularly warm and early spring, when growth starts early in the nursery and seedlings could become too tall and obtain an unbalanced shoot:root ratio (Ministry of Agriculture 1996).
As pointed out by Colombo (2001), the day length during the treatment is important for maintaining prolonged radial growth throughout the SD treatment. Also nutrient supply and the temperature following SD treatment have been shown to influence seedling diameter (Bjørnseth 1977; Fløistad and Granhus 2010), in both cases sub-optimal conditions were associated with reduced diameter growth. In an experiment comparing both timing and duration of SD treatment, Konttinen et al. (2003) observed reduced diameter growth when seedlings were SD treated during four weeks and with an early start of the SD treatment, compared with seedlings treated three weeks or less and with a later start of the treatment. In contrast, Kohmann and Johnsen (2007) reported similar or increased radial growth following SD treatment lasting for one, two or three weeks, compared with no SD treatment.
If sturdier seedlings could be achieved by appropriate manipulation of the growing conditions in nurseries, a precondition for practical application is that bud flush in late summer is avoided. A second bud flush makes seedlings more prone to frost episodes following autumn planting has been associated with a greater risk of infection by grey mould (Botrytis cinerea Pers.:Fr.) and other fungi during winter storage in the nursery (Sandvik 1976; Petäistö 2006). Appropriate timing and duration of the SD treatment is therefore essential to prevent bud flush in late summer (Hawkins et al. 1996; Kohmann and Johnsen 2007; Luoranen et al. 2009). Eastham (1992) observed second bud flush following one week SD treatment in the period late June to mid-July even if one week SD treatment may be sufficient to induce growth cessation. There was a reduction in frequencies of bud flush when the SD treatment was applied later (Eastham 1992). Luoranen et al. (2009) proposed a relationship between temperature sum and the risk of a second bud flush. They found reduced risk of bud flush when the temperature sum was high before the treatment and that low temperature following the SD treatment also reduced the risk of second bud flush.
To produce sturdier seedlings with increased diameter following SD treatment, it would be of considerable value for practical seedling production to determine if there is a narrow window where increased diameter growth is possible within the frame of today’s routines in nurseries, without compromising other seedling quality traits.
The aim of the present study was to identify responses in P. abies seedlings after SD treatment with different starting dates and durations (number of days). Our hypotheses were that (1) early SD treatment results in prolonged diameter growth and thereby sturdier seedlings if SD treatment is applied early compared with seedlings SD-treated later, and (2) seedlings exposed to early SD treatment need a longer duration of the treatment in order to avoid a second bud flush than seedlings treated later in summer.
2 Materials and methods
2.1 Seedling material and experimental conditions
Picea abies seeds, originating in Buskerud County, southeastern Norway (60°N, 10°E, altitude 0–150 m a.s.l.), were sown on 22 May 2002 in limed peat mixed with 25% perlite, in multipot containers (75 cm3 pots, 500 seedlings m–2, 60 pots per container). Germination and the first-year growth phase took place in the greenhouse of a commercial forest nursery in Hokksund (59°46´N, 9°53´E), Buskerud County. Prior to the experiment, on 22 April 2003, seedlings were brought to the daylight phytotron at Ås, Akershus County (59°40´N, 10°51´E) for dormancy release and growing in a controlled environment. Seedlings were grown under natural light conditions and the temperature was gradually increased according to a schedule that simulated the normal spring temperature from daytime/nighttime (DT/NT) 10/6 °C to DT/NT 18/12 °C during 8 weeks of growth. Then, from 20 June onwards, the temperatures were kept at DT/NT 22/18 °C (12 h day and 12 h night gave a daily mean temperature of 20 °C).
Seedlings were exposed to SD treatment (10 h day, 14 h night) for 7, 10, 14 or 17 days with starting dates on 20 June, 27 June, 4 July and 11 July (Fig. 1). Four multipot containers (replicates) were exposed to each of the combinations of duration and starting dates of the SD treatments. In addition four containers received identical growing conditions without SD treatment.
The seedlings were watered and fertilized with a complete nutrient solution (60:40 Pioner 13-4-19 and NH4N03, 1.5 mS cm–1) throughout the experimental treatments. From 4 July the concentration of NH4N03 was reduced (88:12 Pioner 13-4-19 and NH4N03), and from 1 August only Pioner was given.
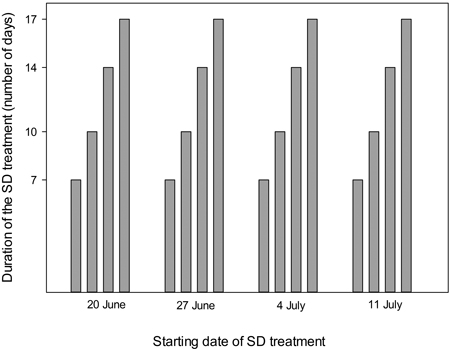
Fig. 1. Time schedule of the experimental short day treatments, with starting dates and durations as indicated.
2.2 Measurements and bud flush assessments
Seedling height was measured weekly on 8 randomly selected seedlings per treatment and replicate from the start of the SD treatment. The same seedlings were measured each time. Stem length was measured from the edge of the pots in the container to the terminal meristem (Mexal and Landis 1990). Every second week, root collar diameter was measured on the same seedlings as used for height growth measurement.
From 4 August and onwards all seedlings were observed twice a week for registration of second bud flush. Upon termination of the experiment, dry weight was determined on 10 seedlings per replicate for seedlings SD treated for 10 and 17 days.
Dry weight was determined separately for roots and above-ground shoots including the stem after oven drying at 65 °C for 48 h.
2.3 Statistical analysis
Analyses of variance were performed using the GLM procedure in SAS (SAS Institute 1989) according to the model:
![]()
where Xijk is the mean of all plants for each combination of the experimental factors considered, µ is the total mean, ti is the fixed effect of treatment duration, sj is the fixed effect of starting date, tsij is the interaction between treatment duration and starting date and ck is the random effect of container (replicate) and eijk is the experimental error.
Separate analyses of treatment duration were performed to allow a comparison that included the non-treated control. The statistical significance of difference between individual treatments was assessed by the Bonferroni method (SAS Institute 1989).
The effect of SD treatment on second bud flush was assessed by calculating large-sample 95% confidence intervals (Agresti 1996) for the frequency of seedlings with a second bud flush upon termination of the experiment. The treatments were considered significantly different if confidence intervals did not overlap.
3 Results
3.1 Seedling height and root collar diameter
Height growth of the seedlings was significantly affected by SD treatment (p < 0.0001). The earliest starting date, on 20 June, resulted in significantly shorter seedlings than all other treatments (p < 0.0001) (Fig. 2A and Fig. 3). Also, seedlings with SD-treatment starting on 27 June had shorter height growth than seedlings with a later start of the treatment (p < 0.0001).
Seedlings without SD treatment had significantly more height growth than seedlings given SD treatment (p = 0.0008 for 7 days and p = 0.0001 for 10–17 days of treatment) (Fig. 3). When comparing the seedlings’ height within starting dates of the SD treatment, no significant differences appeared for different durations of SD treatment (Fig. 2A).
Diameter growth was significantly less in seedlings exposed to 14 or 17 days of SDj treatment than in seedlings exposed to 7 or 10 days of SD treatment (p < 0.0001) (Fig. 2B). The earliest starting date of the SD treatment, on 20 June, resulted in significantly more diameter growth compared with the two latest starting dates (p < 0.0001; Fig. 2B).
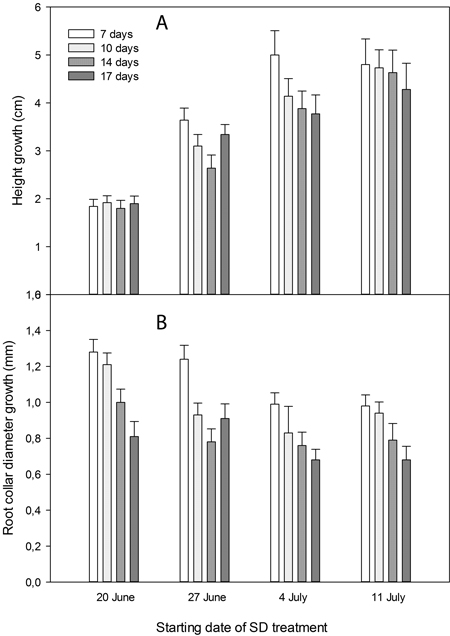
Fig. 2. Height (A) and root collar diameter (B) growth from start of the experimental treatments on 20 June until termination of the experiment on 1 September in Picea abies seedlings exposed to SD treatment with different starting dates and durations as indicated.
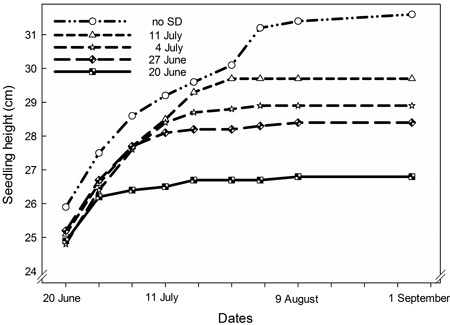
Fig. 3. Seedling height in Picea abies seedlings exposed to SD treatment with different starting dates as indicated.
3.2 Dry weight
Untreated seedlings had the highest average shoot dry weight (p < 0.0001). There was a tendency, however non-significant, towards higher shoot dry weight in seedlings with late and short SD treatments, compared with earlier and shorter SD treatments (Table 1). No differences in root dry weight appeared between any of the treatments.
| Table 1. Dry mass of shoots and roots of Picea abies seedlings exposed to short day treatment with different starting dates and durations. Values followed by the same letter are not significantly different (p > 0.05). | |||
| Starting date | Duration (days) | Dry weight shoots (g seedling–1) | Dry weight roots (g seedling–1) |
| 20 June | 10 | 2.74 ad | 0.98 a |
| 20 June | 17 | 2.47 a | 1.00 a |
| 27 June | 10 | 2.62 a | 1.02 a |
| 27 June | 17 | 2.57 a | 0.97 a |
| 4 July | 10 | 2.81 ad | 1.01 a |
| 4 July | 17 | 2.82 ad | 1.03 a |
| 11 July | 10 | 3.29 b | 1.07 a |
| 11 July | 17 | 3.07 bd | 0.98 a |
| Control | 0 | 3.95 c | 1.20 a |
| Note: Dry weight was only determined for seedlings SD-treated for 10 and 17 days. | |||
3.3 Second bud flush
A second bud flush occurred only in seedlings exposed to SD treatment with starting day 20 or 27 of June (Fig. 4) and 7–14 days of duration. The highest frequencies of second bud flush were observed among the lateral buds. Regardless of starting date, 17 days of SD treatment was always sufficient to prevent a second bud flush. A total of 14% and 4% of seedlings appeared with second bud flush when SD treatment of 7 days duration started on 20 June and 27 June, respectively. When 10 days of SD treatment started on the same dates the occurrence of second bud flush was only 6% and 1%, respectively. Following 14 days of SD treatment starting on 20 June, merely 1% of the seedlings appeared with a second bud flush. Seven days of SD treatment starting 20 June resulted in significantly higher frequencies of bud flush than both 14 days of SD treatment starting 20 June and 10 days of SD treatment starting 27 June (p = 0.05).
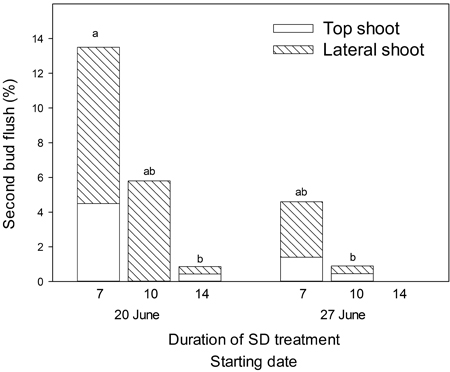
Fig. 4. Amount of Picea abies seedlings with second bud flush following SD treatment with different durations and starting dates as indicated. Only dates and durations leading to second bud flush are shown.
4 Discussion
SD treatment is used in forest nurseries to stop height growth and thereby induce growth cessation (Dormling et al. 1968; Colombo et al. 2001). Seedling morphology also influences the quality of seedlings and their performance after planting (Thompson 1985; Mattsson 1997). Sturdy seedlings are especially needed on sites prone to attack by pine weevils (Hylobius spp.). Therefore, we wanted to explore the possibility to influence the diameter growth of seedlings following SD treatment. Based on previous research, we expected root collar diameter to have a slower reaction to SD treatment than height growth (Dormling et al. 1968; Heide 1974a; Bjørnseth 1977). We therefore hypothesized that it should be possible to utilize SD treatment to stop height growth, while at the same time maintaining diameter growth sufficiently long to improve the sturdiness of the seedlings (Kohmann and Johnsen 2007).
This study indicate that for root collar diameter growth, the duration of the SD treatment is more important that the timing, in accordance with the findings of Turner and Mitchell (2003) in Douglas-fir (Pseudotsuga menziesii Mirb. Franco). However, also timing influenced on diameter growth as the earliest start of the SD treatment resulted in increased diameter growth compared with the two latest staring dates. Colombo (2001) pointed out the importance of maintaining a relatively long daylength during the SD treatment to allow for photosynthesis and diameter growth. Bigras and D’Aoust (1993) also showed reduced root collar diameter in white spruce (P. glauca (Moench) Voss) when the photoperiod during the SD treatment decreased. Our results indicate that the duration of the SD treatment affects diameter growth in a similar manner as the actual photoperiod during treatment (Konttinen et al. 2003).
Seedlings without SD treatment achieved the largest average root collar diameter in our experiment. A similar result was obtained by Konttinen et al. (2007), but our findings are in contrast to Kohmann and Johnsen (2007), who found similar or smaller root collar diameter in untreated compared to SD treated seedlings. The contradictory results could possibly be explained by a smaller root volume and higher density (Von Wuhlisch and Muhs 1987) of the relatively taller seedlings in the outdoor nursery experiment by Kohmann and Johnsen (2007), compared to our seedlings. Another possible explanation may be more favourable light and fertilization conditions in the present experiment (Bjørnseth 1977).
This study also confirmed the rapid and uniform height growth cessation in P. abies following SD treatment as documented previously (Dormling et al. 1968; Heide 1974a). Earlier start of SD treatment resulted in shorter seedlings in our experiment. This illustrates how SD treatment may be used to stop height growth in the nursery.
When a second bud flush appear in seedlings in late summer, they will be prone both to frost damage and fungus infection (Sandvik 1976; Petäistö 2006). This study showed a clear relationship between timing of the SD treatment and frequencies of second bud flush. Only when the SD treatment started in June did second bud flush occur. This is in accordance with earlier findings (Eastham 1992; Kohmann and Johnsen 2007; Fløistad and Granhus 2010). We have previously reported on easier flushing of lateral buds compared to apical buds (Fløistad and Granhus 2010). This reaction is possibly due to a more shallow level of dormancy in lateral buds (Junttila et al. 2003).
The gradually declining daylengths after summer solstice provide a logical explanation for the lower frequencies of second bud flush following late SD treatment. However, a temperature-driven response, as proposed by Luoranen et al. (2009), could also be important and possibly interact with the photoperiod. By analysing results from three subsequent years they proposed that the risk of reflushing increased if the temperature sum at the start and at the end of the SD treatment was below or above a certain level, respectively. This may also explain the different level of reflushing in our experiment compared with Kohmann and Johnsen (2007), both performed with identical seed lots.
In addition to timing of the SD treatment, the duration is important to avoid a second bud flush, especially when combined with an early start of the SD treatment (Eastham 1992; Kohmann and Johnsen 2007; Luoranen et al. 2009). In our experiment no or only limited bud flush occurred following 17 and 14 days of SD treatment, respectively. Practical implications could be recommendations of longer duration of the SD treatment if the treatment starts early, although our findings imply that this will be at the expense of sustained diameter growth. Based on the findings of Luoranen et al. (2009), considering the risk of second bud flush may be even more important if the temperature sum before start of the treatment is low, or if the temperature sum after the SD treatment is high, e.g. when seedlings are planted late in the summer or early in the autumn.
An important issue not addressed here is the possibility of earlier spring bud break in SD treated seedlings, which could be detrimental following early planting on frost prone sites (Heide 1974b; Bigras and D’Aoust 1992; Fløistad and Granhus 2010). Conflicting results exists, however, on how different timing of the SD treatment influence bud break (Konttinen et al. 2003; Rostad et al. 2006; Fløistad and Granhus 2010). This should be further studied to obtain sufficient information for guiding nurseries on SD routines.
Acknowledgements
The support for this study by the Research Council of Norway through projects no 153738/140 is gratefully acknowledged. We also thank Marit Helgheim for excellent technical assistance and two anonymous reviewers for constructive comments. The Norwegian Institute for Agricultural and Environmental Research, the Norwegian Forest and Landscape Institute and the Norwegian foundations “Skogbrukets Utviklingsfond” and “Skogtiltaksfondet” are also acknowledged for their support.
References
Agresti A. (1996). An introduction to categorical data analysis. John Wiley & Sons, New York. 290 p.
Bigras F.J., D’aoust A. (1992). Hardening and dehardening of shoots and roots of containerized black spruce and white spruce seedlings under short and long days. Canadian Journal of Forest Research 22: 388–396. http://dx.doi.org/10.1139/x92-051.
Bigras F.J., D’aoust A. (1993). Influence of photoperiod on shoot and root frost tolerance and bud phenology of white spruce seedlings (Picea glauca). Canadian Journal of Forest Research 23: 219–228. http://dx.doi.org/10.1139/x93-029.
Bjørnseth I.-P. (1977). Cessation of cambial activity in Norway spruce (Picea abies (L.) Karst.), its relation to natural daylength, temperature and nutrition. Department of Forest Genetics, Royal College of Forestry, Stockholm, Sweden, Research Notes 27: 32–39.
Colombo S.J., Menzies M.I., O’Reilly C. (2001). Influence of nursery cultural practices on cold hardiness of coniferous forest tree seedlings. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. Kluwer Academic Publishers. p. 223–252.
Dormling I., Gustafsson Å., Von Wettstein D. (1968). The experimental control of the life cycle in Picea abies (L.) Karst. I. Some basic experiments on the vegetative cycle. Silvae Genetica 17: 44–64.
Eastham A.M. (1992). Timing of backout application to regulate height in sitka x white spruce hybrid 1+0 container-grown seedlings In: 11th annual meeting of forest nursery association of B.C. Prince George, B.C., Canada. p. 86–92.
Fløistad I.S., Granhus A. (2010). Bud break and spring frost hardiness in Picea abies seedlings in response to photoperiod and temperature treatments. Canadian Journal of Forest Research 40: 968–976. http://dx.doi.org/10.1139/X10-050.
Hawkins C.D.B., Eastham A.M., Story T.L., Eng R.Y.N., Draper D.A. (1996). The effect of nursery blackout application on Sitka spruce seedlings. Canadian Journal of Forest Research 26: 2201–2213. http://dx.doi.org/10.1139/x26-249.
Heide O.M. (1974a). Growth and dormancy in Norway spruce ecotypes (Picea abies). I. Interaction of photoperiod and temperature. Physiologia Plantarum 30: 1–12. http://dx.doi.org/10.1111/j.1399-3054.1974.tb04983.x.
Heide O.M. (1974b). Growth and dormancy in Norway spruce ecotypes. II. After-effects of photoperiod and temperature on growth and development in subsequent years. Physiologia Plantarum 31: 131–139. http://dx.doi.org/10.1111/j.1399-3054.1974.tb03117.x.
Junttila O., Nilsen J., Igeland B. (2003). Effects of temperature on the induction of bud dormancy in ecotypes of Betula pubescens and Betula pendula. Scandinavian Journal of Forest Research 18: 208–217. http://dx.doi.org/10.1080/02827581.2003.9728291.
Kohmann K., Johnsen Ø. (2007). Effects of early long-night treatment on diameter and height growth, second flush and frost tolerance in two-year-old Picea abies container seedlings. Scandinavian Journal of Forest Research 22: 375–383. http://dx.doi.org/10.1080/02827580701520486.
Konttinen K., Luoranen J., Rikala R. (2007). Growth and frost hardening of Picea abies seedlings after various night length treatments. Baltic Forestry 13: 140–148.
Konttinen K., Rikala R., Luoranen J. (2003). Timing and duration of short-day treatment of Picea abies seedlings. Baltic Forestry 9: 2–9.
L’hirondelle S.J., Simpson D.G., Binder W.D. (2006). Overwinter storability of conifer planting stock: operational testing of fall frost hardiness. New Forests 32: 307–321. http://dx.doi.org/10.1007/s11056-006-9005-8.
Luoranen J., Konttinen K., Rikala R. (2009). Frost hardening and risk of a second flush in Norway spruce seedlings after an early-season short-day treatment. Silva Fennica 43: 235–247.
Mattsson A. (1997). Predicting field performance using seedling quality assessment. New Forests 13: 227–252. http://dx.doi.org/10.1023/A:1006590409595.
Mexal J.G., Landis T.D. (1990). Target seedling concepts: height and diameter. In: Rose R., Campbell S.J., Landis T.D. (eds.). Proceedings, Western Forest Nursery Association, 1990 August 13–17, Roseburg, OR. USDA Rocky Mountain Forest and Range Experiment Station, General Technical Report RM-200. p. 17–35.
Ministry of Agriculture. (1996). Forskrift om skogfrø og skogplanter. Oslo, Norway. 5 p.
Petäistö R.-L. (2006). Botrytis cinerea and Norway spruce seedlings in cold storage. Baltic Forestry 11: 24–33.
Rostad H., Granhus A., Fløistad I.S., Morgenlie S. (2006). Early summer frost hardiness in Picea abies seedlings in response to photoperiodic treatment. Canadian Journal of Forest Research 36: 2966–2973. http://dx.doi.org/j10.1139/x06-167.
Sandvik M. (1976). Vinterstyrke hos gran. I. Effect av opptakstid, nedkjøling og opptining ved fryselagring av granplanter. [Winter vigour in Picea abies (L.) Karst. I. Effects of lifting date, cooling and thawing by cold storage of seedlings]. Meddelelser fra Norsk institutt for skogforskning 32: 337–355.
SAS Institute. (1989). SAS/STAT© User’s Guide. Cary, NC: SAS Institute Inc. 846 p.
Thompson B.E. (1985). Seedling morphological evaluation – what you can tell by looking. In: Duryea M.L. (ed.). Evaluating seedling quality: principles, procedures and abilities of major tests. Proceedings of the workshop held October 16–18, 1984. Forest Research Laboratory, Oregon State University, Corvallis. p. 59–71.
Turner J., Mitchell S.J. (2003). The effect of short day treatments on containerized Douglas-fir morphology, physiology and phenology. New Forests 26: 279–295. http://dx.doi.org/10.1023/A:1024406704381.
Von Wuhlisch G., Muhs H.J. (1987). Effect of spacing on growth, especially predetermined and free shoot growth of Norway spruce (Picea abies (L.) Karst.). Silvae Genetica 36: 72–76.
Total of 26 references

