Ophiostomatoid fungi and their roles in Quercus robur die-back in Tellermann forest, Russia
Selochnik N. N., Pashenova N. V., Sidorov E., Wingfield M. J., Linnakoski R. (2015). Ophiostomatoid fungi and their roles in Quercus robur die-back in Tellermann forest, Russia. Silva Fennica vol. 49 no. 5 article id 1328. https://doi.org/10.14214/sf.1328
Highlights
- Dominant ophiostomatoid fungi associated with Q. robur in the post-outbreak region of oak die-back were investigated
- Ophiostoma quercus was the most commonly encountered fungus
- This is the first report of O. grandicarpum from Russia
- The results of preliminary pathogenicity experiments demonstrate that fungi investigated in this study are unlikely to play causal role in oak die-back
Abstract
Several eastern European countries have reported outbreaks of oak die-back during the 1980’s. Species of Ophiostoma Syd. were isolated from diseased trees and have been suggested to be the possible causal agents of the die-back, but this view have generally not been accepted. In order to monitor the post-outbreak region of oak die-back and to consider the possible role of Ophiostoma spp. in the syndrome, research has been conducted in the Tellerman forest, Voronezh region, Russia between 2005 and 2011. Our study resulted in the isolation of ophiostomatoid fungi from Quercus robur L. trees displaying external signs of desiccation. Fungi were identified based on morphological characteristics and DNA sequence comparisons. Three species of Ophiostoma were identified including O. grandicarpum (Kowalski & Butin) Rulamort, a species closely related to O. abietinum Marm. & Butin, O. fusiforme Aghayeva & M.J. Wingf. and O. lunatum Aghayeva & M.J. Wingf. representing a poorly understood species complex, and most commonly O. quercus (Georgev.) Nannf. Pathogenicity of these fungi was tested using artificial inoculations on Q. robur trees. The fungi were shown to be non-pathogenic and unlikely to play any role in oak die-back. These fungi are most likely only components in a complex of abiotic, biotic and anthropogenic factors that have contributed to a die-back of Quercus spp. in Russia.
Keywords
Ophiostomatales;
Ophiostoma;
oak die-back;
pathogenicity
- Selochnik, Forest Science Institute of RAS, Uspenskoe 143030, Moscow Region, Russia E-mail lenelse@yandex.ru
- Pashenova, V.N. Sukachev Institute of Forest SB RAS, Krasnoyarsk 660036, Russia E-mail pasnat@ksc.krasn.ru
- Sidorov, Department of Forest Protection and Game Management, St. Petersburg State Forest Technical University, St. Petersburg 194021, Russia E-mail sidorov_evgeny@mail.ru
- Wingfield, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, 0002 Pretoria, South Africa E-mail mike.wingfield@up.ac.za
-
Linnakoski,
Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, 0002 Pretoria, South Africa; Department of Forest Sciences, University of Helsinki, P.O. Box 27, FI-00014 University of Helsinki, Finland
 http://orcid.org/0000-0002-3294-8088
E-mail
riikka.linnakoski@helsinki.fi
http://orcid.org/0000-0002-3294-8088
E-mail
riikka.linnakoski@helsinki.fi

Received 3 March 2015 Accepted 27 August 2015 Published 18 September 2015
Views 173506
Available at https://doi.org/10.14214/sf.1328 | Download PDF

1 Introduction
Severe outbreaks of oak die-back and the potential spread of the disease was of great concern to Eastern European countries in the 1980’s. Research focused on a search of the primary causal agents and one of the hypotheses suggested that fungi were responsible for the problem (OEPP/EPPO 1983). In this regard, species of Ophiostoma (Ascomycota) were isolated and suggested to be the causal agents (Sczerbin-Parfenenko 1953; Cech et al. 1990).
The oak die-back in Eastern Europe, often described in early publications as ‘vascular mycosis of oak’, was first recorded in Yugoslavia in 1926 (Georgevitch 1926, 1927). Later it was discovered in other European countries and in the USA (Georgescu et al. 1948; Petrescu et al. 1974). In Russian national literature, the die-back where fungi were associated was first mentioned in 1950’s (Sczerbin-Parfenenko 1953). Mass oak die-back associated with fungal agents has been reported in several southern regions of Russia (Kryukova and Balder 1993), and in the former Soviet Republics (Guseinov 1984). In the Voronezh region, oak die-back where Ophiostoma spp. were associated with the symptoms was first discovered and described in 1957 by Ivanchenko. The disease in the Voronezh region was subsequently reported in other studies (Minkevich 1962, 1964; Kuzmichev 1983). An extensive review of the literature concerning oak die-back in the USSR and the role of ophiostomatoid fungi in this syndrome was published by Oleksyn and Przybil (1987).
Reports of ophiostomatoid species associated with oak die-back in Europe (former Yugoslavia and Czechoslovakia, Romania, France, Bulgaria, Poland, Austria) include Ophiostoma quercus (Georgev.) Nannf., one of the most commonly occurring and widespread sap-stain fungi found on hardwoods. This species has been the subject of a relatively long history of taxonomic confusion with several similar hardwood-infecting species commonly including Ophiostoma piceae (Münch) Syd. (Brasier 1993). Recent in-depth studies based on DNA sequence analyses have shown conclusively that O. quercus and O. piceae represent distinct taxa that also differ ecologically (Harrington et al. 2001; de Beer et al. 2003; Grobbelaar et al. 2009; Linnakoski et al. 2010). Ophiostoma quercus resides in the O. ulmi-complex together with other hardwood-infesting Ophiostoma spp., while O. piceae is mostly isolated from conifer hosts (de Beer and Wingfield 2013).
Several species names have been listed in the literature reporting oak die-back-associated ophiostomatoid fungi. Recent studies have shown that the following species are the synonyms of O. quercus: Ceratostomella quercus Georgev., Ceratocystis querci (Georgev.) C. Moreau, Ceratostomella fagi W. Loos, Ophiostoma fagi (W. Loos) Nannf., Ceratocystis fagi (W. Loos) C. Moreau, Ophiostoma roboris Georgescu & Teodoru, Ceratocystis roboris (Georgescu & Teodoru) Potl., Graphium roboris Georgescu, Teodoru & Badea, Pesotum roboris (Georgescu, Teodoru & Badea) Grobbelaar, Z.W. de Beer & M.J. Wingf., Hyalodendron roboris Georgescu & Teodoru and Sporothrix roboris (Georgescu & Teodoru) Grobbelaar, Z.W. de Beer & M.J. Wingf. (Grobbelaar et al. 2009; de Beer et al. 2013). Ophiostoma kubanicum Sczerbin-Parfenenko (synonyms Graphium kubanicum Sczerbin-Parfenenko, Verticillium kubanicum Sczerbin-Parfenenko and Ceratocystis kubanica (Sczerbin-Parfenenko) Potlajchuk has also been reported from oak in the former USSR but the species was never validly described and is treated as nomen invalidum and excluded from Ophiostoma (Grobbelaar et al. 2009) and is also not considered in this study. Other than the confusion arising from the occurrence of many synonyms of O. quercus, the literature has also been confused due to the use of the epithet ‘querci’ as opposed to the correct form ‘quercus’ argued by de Beer et al. (2003).
While O. quercus or its synonyms has been the most frequently reported fungus in literature pertaining to oak die-back in Eastern Europe, other ophiostomatoid (Wingfield et al. 1993) fungi have been found in association with this disease. These include O. valachicum Georgescu, Teodoru & Badea (synonyms Rhinotrichum valachicum Georgescu, Teodoru & Badea and Ceratocystis valachicum Georgescu, Teodoru & Badea) Potl.), O. introcitrinum (Olchow. & J. Reid) Hausner, J. Reid & Klassen, C. moniliformis (Hedgc.) C. Moreau, O. stenoceras (Robak) Nannf., O. grandicarpum (Kowalski & Butin) Rulamort, O. piliferum (Fr. : Fr.) Syd. and O. proliferum (Kowalski & Butin) Rulamort (Kowalski and Butin 1989; Cech et al. 1990).
The post-outbreak monitoring of oak die-back in Russia commenced in 1983 in the Tellerman Experimental Forest (TEF) of the Russian Academy of Science (RAS) Forest Institute, Voronezh region, Russia (Selochnik and Kondrashova 1989). The symptoms have been recorded in all different forest types found in the Tellerman forest, extending to Ryazan, Tula, Lipetsk, Tambov, Belgorod and Voronezh regions of the Central Russian Plain (Osipov and Selochnik 1989). Monitoring has included recording potential internal symptoms of vascular discoloration (dark spots, streaks and rings in cross sections of the branches and trunks, and blue-stain of the sapwood). External signs have been absent or rarely present in the form of yellowing or light reddening of leaves, sometimes slightly curled and somewhat shaggy crowns on trees. These mild symptoms of oak die-back have been present in the TEF of the Voronezh region and other abovementioned areas during our previous studies between 1983–2011 (Selochnik 1998; Selochnik 2000; Selochnik and Pashenova 2007), but mass die-back has never been observed.
Our hypothesis is that in their native range, ophiostomatoid fungi are common wood-colonizing, saprophytic fungi that do not have major importance in die-back of Quercus spp. in Russia. The aim of this study was to isolate and identify the dominant ophiostomatoid species from Q. robur trees in the post-outbreak region of oak die-back in the Tellerman forest of Russia, and to consider, in preliminary tests, their potential pathogenicity to these trees.
2 Materials and methods
Studies were carried out between 2005–2011 and included three phases: 1) a long-term sampling of oak tissue that included isolation of representative fungi to pure culture; 2) morphological and DNA-sequence based identification of the isolated fungi; 3) preliminary artificial inoculations to consider the pathogenicity of the isolated fungi.
2.1 The study area
The Tellerman Experimental Forest (TEF) has served for biological research since 1945. It is a polygon shaped area with a length of 65 km and the width varying between 3 and 16 km. The total TEF area is 2027 hectares; the geographic coordinates 50°58´N, 41°43´E; the average annual air temperature 6.6 °C; annual precipitation in the years of the present studies varied between 300–400 mm, with multiple dry periods annually. The climate is moderately continental; the soils are predominantly of grey forest type (more productive) and solonets (less productive). The principal forest types in TEF are upland, flood-land, hillslope and solonets (Osipov et al. 1989).
Samples were collected from different aged oak trees during reconnaissance studies in the TEF area to examine the presence of ophiostomoid fungi (see 2.2). A sample plot was established in the TEF area to conduct the inoculation experiments. The plot consisted of a 10- to 12-year-old, natural oak tree stand situated on the Khoper river floodland, along the river bank. The selection of trees for different treatments (including a control group) was carried out by randomization (drawing lots).
2.2 Collection of fungal samples
Fungal isolates were obtained from cuttings and increment cores taken from Q. robur wood from 2005 to 2009. Samples were collected from oaks of different ages (60–100-year-old) showing external signs of desiccation (crown thinning, leaf yellowing, presence of branches with chlorotic or dead leaves and epicormic, sprouts on the trunks). Samples from the phloem and sapwood were taken from trees at breast height. The wood samples were placed into moist chambers (Petri dishes with moistened filter paper) and maintained at room temperature for four weeks to induce fungal sporulation. Moist chambers containing samples were examined weekly using a SBM-9 dissection microscope (LZOS, JSC, Russia).
Initial identification of fungal species was done based on morphological characteristics of asexual and sexual structures. Representative strains of each species were isolated in pure cultures. Fungi sporulating on incubated wood tissue were transferred to 2% malt extract agar (MEA; 20 g Difco® malt extract and 15 g agar [Helicon, Russia] and 1 L water) and potato dextrose agar (PDA) produced by Lab-BioMed Ltd., Russia. One representative strain of each isolated ophiostomatoid fungus was subjected to DNA sequence-based identification and these strains are stored at the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa.
2.3 DNA sequencing
Fungal isolates subjected to DNA sequence-based identification were grown on MEA in 70 mm Petri dishes. DNA was extracted using PrepMan Ultra Sample preparation reagent (Applied Biosystems, Foster City, CA, USA) and the same protocols described by Linnakoski et al. (2008).
The internal transcribed spacer regions ITS 1 and 2, including the 5.8S gene, and partial β-tubulin gene regions were amplified and sequenced. The ITS region was amplified using primers ITS1-F (Gardes and Bruns 1993) and ITS4 (White et al. 1990). The partial β-tubulin gene region was amplified using primers T10 (O’Donnell and Cigelnik 1997) and Bt2b (Glass and Donaldson 1995).
Amplification of the gene regions was performed in 25 μL reaction mixture. The reaction mixture contained 0.15 μL of MyTaqTM DNA Polymerase (5 U μL–1) (Bioline, Massachusetts, USA), 2.5 μL of MyTaqTM Reaction Buffer (5) containing dNTPs, MgCl2 and enhancers for the optimal performance (supplied with the enzyme), and 0.50 µL of each primer (10mM) (Whitehead Scientific Ltd, Cape Town, South Africa). PCR reactions were performed using an ABI 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) with the following conditions: an initial denaturation step at 95 °C for 2 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C and 1 min at 72 °C, and a final chain elongation at 72 °C for 7 min. Amplified products were purified using the Exo-SAP protocol: 20 μL of the PCR product was mixed with 8 μL of Exo-SAP (5 μL of Exonuclease I (20 U µL–1) (Fermentas,Vilnius, Lithuania) and 100 µL of Shrimp Alkaline Phosphatase (1 U µL–1) (Roche Diagnostics, Indianapolis, USA) in 1000 µL reaction mixture) and incubated at 37 °C for 15 minutes and following immediate incubation at 80° for 15 minutes.
The cleaned PCR products were sequenced with the BigDye v3.1 Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) on the ABI Prism 377 Autosequencer (Applied Biosystems, Foster City, CA, USA) at the DNA Sequencing Facility of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria. The primers used for sequencing the ITS gene region were the same as those used for PCR. For sequencing the partial β-tubulin gene, the T10 primer was replaced with the primer Bt2a (Glass and Donaldson 1995).
The consensus sequences were determined using the program Geneious R6 for MacIntosh (Biomatters Ltd, Auckland, New Zealand). Datasets were compiled in Molecular Evolutionary Genetic Analysis (MEGA) v6 (Tamura et al. 2013). Sequence alignments were performed with the online version of MAFFT v7 (Katoh and Standley 2013), using the FFT-NS-i option with a gap opening penalty of 1.53 and an offset value of 0.00. The sequences obtained in this study were deposited in GenBank and their accession numbers are presented in the Table 1.
| Table 1. Morphological characteristics of ophiostomatoid fungi found in conductive tissues of Quercus robur in the Tellerman forest, Russia. Results are based on 30–50 measurements of each taxonomically informative morphological structure. | |||
| Characteristics | O. fusiforme-like | O. grandicarpum | O. quercus |
| Perithecia | |||
| base diameter (µm) | 100–250 | 350–500 | 160–220 |
| neck length (µm) | 350–800 | 2000–8000 | 930–1600 |
| Ostiolar hyphae | present | absent | present |
| Ascospores | |||
| shape | allantoid, no sheath | orange section, possibly with sheath | allantoid, no sheath |
| size (µm) | (4.5–5.0) × (1.0–1.5) | (4.0–5.0) × (1.5–2.0) | (3.9–5.2) × (1.3–2.0) |
| Anamorph | sporothrix-like | sporothrix-like | sporothrix- and pesotum-like |
| Growth rate on malt extract agar (mm day–1) | 1.3 ± 0.2 | 1.3 ± 0.2 | 4.0 ± 0.4 |
| Colony morphology | snow-white, fluffy, ascending | light-grey, no aerial mycelia | initially white, darkening with age |
| Substrate | phloem аnd sapwood | phloem | phloem аnd sapwood |
| Culture collection no. | CMW41130 | CMW41131 | CMW41129 |
| GenBank acc. no | |||
| ITS | KP289352 | KP289353 | KP289351 |
| β-tubulin | KP289355 | KP289356 | KP289354 |
Phylogenetic analyses were performed using three different methods: maximum likelihood (ML), maximum parsimony (MP) and Bayesian inference (BI). ML analyses were performed using RAxML v7.0.4 (Stamakis 2014) run on the CIPRES Science Gateway v3.3 (Miller et al. 2010) employing the GTR substitution matrix and a rapid bootstrap analysis (Stamakis et al. 2008) to search for the best-scoring ML tree. The number of bootstrap replicates was estimated using the bootstopping criterion implemented in RAxML (Pattengale et al. 2010). MP analyses were conducted using (MEGA) v6 (Tamura et al. 2013), using a bootstrap test with 1000 replicates and tree-bisection-recognition (TBR) branch swapping. Gaps and missing data included in the analyses. BI analyses based on a Markov Chain Monte Carlo (MCMC) were carried out with MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003). The best fitting evolutionary models for each data set were determined using MrModeltest v2.3 (Nylander 2004) based on the Akaike Information Criterion (AIC). The MCMC chains were run for five million generations using the sample frequency of 100 (resulting in 50000 trees). Burn-in values were calculated for the respective data sets, and all sampled trees having lower than the burn-in values were discarded. The remaining trees were used to construct majority rule consensus trees.
2.4 Pathogenicity tests
The inoculation experiment commenced at the beginning of August 2010. Pathogenicity of the isolated fungi was tested in field conditions using artificial inoculations on young (10- to 12-year-old), asymptomatic Q. robur trees growing in the floodplain of the Khoper river in the TEF. The tree heights and stem diameters were measured at the beginning of the inoculation experiment. Oak trees were similar in height and stem diameter. These parameters varied from 2.0 to 5.5 m (height) and from 3.0 to 6.0 cm (stem diameter at breast height).
Three ophiostomatoid species originating from the TEF were used in the inoculation experiments (Table 1). These fungi included three strains of each of the two ophiostomatoid species that were found in high occurrence in the examined wood samples (Table 2). In addition, a single strain of a fungus that was never observed in TEF earlier was included in the pathogenicity tests. The test fungi were cultivated on MEA at 22–24 °C prior to the experiment. Pathogenicity tests were performed using agar blocks (10 × 10 mm) with mycelium cut from the edges of 7- to 10-day-old cultures. The agar blocks covered with mycelium were placed in equivalent size (15 × 30 mm) sized rectangular wounds made by lifting the bark from the stems of young trees (10- to 12-year-old, 2–5 m in height) to expose the cambium but without removing the bark disc. The tree diameters at the inoculation points (120–150 cm height) were 3–6 cm. In total 27 trees were inoculated in the experiment. Each test strain was inoculated on three replicate trees (in total 21 trees) and six trees served as controls. The control trees were either not inoculated (three trees) or inoculated with blocks of sterile agar (three trees). The inoculation points were covered with the removed portion of the bark and the wounds were sealed with adhesive tape.
| Table 2. Number of ophiostomatoid fungi in collected Quercus robur samples in Tellerman forest. In total of 286 wood samples were investigated during 2005–2009. More than one ophiostomatoid species were possible to find in the same wood sample. | ||
| Fungal species | No. of wood samples with fungi | Percentage of fungi (%) |
| In total | ||
| Ophiostoma grandicarpum | 15 | 5.2 |
| O. fusiforme-like | 61 | 21.3 |
| O. quercus | 93 | 32.5 |
| Species present in the same sample | ||
| O. grandicarpum alone | 12 | 4.2 |
| O. fusiforme-like alone | 31 | 10.8 |
| O. quercus alone | 65 | 22.7 |
| O. grandicarpum + O. fusiforme-like | 2 | 0.7 |
| O. grandicarpum + O. quercus | 0 | 0 |
| O. fusiforme + O. quercus | 27 | 9.4 |
| O. grandicarpum + O. fusiforme-like + O. quercus | 1 | 0.4 |
| Total | 138 | 48.2 |
A preliminary examination of the inoculations was made after approximately six weeks (mid-September 2010) and the study was terminated after approximately ten months (early May 2011). At this time the tree condition was assessed and the sizes of the lesions (length and width) were recorded. To meet the requirements of Koch’s postulates, isolations were made from the inoculation sites when the experiment was terminated.
2.5 Statistical analyses
The results of the pathogenicity experiment were statistically tested. The lesion length and width that resulted from the fungal colonization of the phloem were used as a measure to evaluate the fungal virulence. The assumption was that more virulent the fungi cause longer lesions. Because of small sample size (n < 10), unequal sampling and unknown sampling distribution, the Mann-Whitney test was used to determine the significance of lesion size (length and width) differences between different fungal species. The statistical analyses were performed using STATISTICA 8 (StatSoft).
3 Results
3.1 Occurrence of fungi in wood samples
Ophiostomatoid fungi were present in approximately 50% of the 286 wood samples collected over the five-year study period (Table 2). Within two weeks of incubation in the moist chambers, morphological structures typical to Ophiostoma spp. appeared on the wood surfaces. Cultures transferred on MEA and PDA all produced mononematous sporothrix-like and synnematous pesotum-like conidiophores. Sexual fruiting structures (ascomata) were observed after 4–6 week growth on MEA.
Morphological studies revealed that three species of ophiostomatoid fungi were present in the phloem and sapwood of Q. robur and these species were isolated in pure culture (Table 1). Preliminary identification based on morphological characteristics showed that two of the fungi represented O. grandicarpum and O. quercus. Morphological characteristics of the remaining species did not allow clear morphological identification. Preliminary identification of these fungi based on morphology suggested that the remaining fungus represented species similar to Ophiostoma fusiforme Aghayeva & M.J. Wingf.
Ophiostoma quercus and O. fusiforme-like were the most frequent species in the wood samples inspected (32.5% and 21.3%, respectively), whereas O. grandicarpum was found in 15 (5.2%) of the samples (Table 2). More than one ophiostomatoid species often occurred on the same wood sample, but O. fusiforme–O. quercus combination was the most common (Table 2).
3.2 Molecular identification of fungi
The DNA sequence data were analyzed in three separate datasets. The aligned DNA sequence datasets consisted of 755 characters (including the gaps) for the ITS region, and 336 and 276 characters (including the gaps) for the β-tubulin datasets, respectively. ITS data provided the position of the species in the species complexes (Fig. 1) of the Ophiostomatales (de Beer and Wingfield 2013). The phylogram based on the ITS sequences showed that one of the fungal species obtained in this study resided in the O. ulmi complex in Ophiostoma sensu stricto. The other two species resided in Ophiostoma sensu lato with one being a member of the Sporothrix schenckii–O. stenoceras complex. The DNA sequence data for the remaining species matched data in GenBank for O. grandicarpum (AJ293884). Based on the ITS data, this species is distinct from currently known species in Ophiostoma sensu lato as well as any other for which sequences are currently available in GenBank.
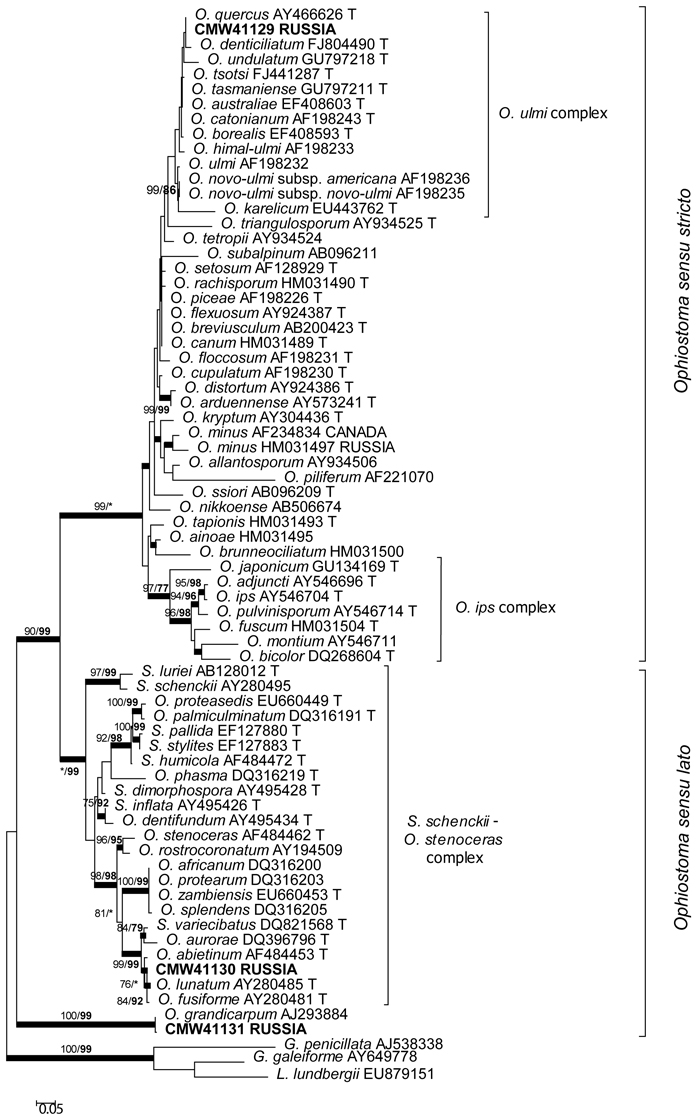
Fig. 1. Phylogram obtained from ML analyses of the ITS regions. Isolate numbers of sequences obtained in this study are printed in bold type. The bootstrap support values for ML (normal type) and MP (bold type) above 70% are indicated at the nodes. Posterior probabilities (above 90%) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 70%. T = ex-type isolates. Scale bar = total nucleotide difference between taxa.
The β-tubulin datasets were used to resolve the species level relationships (Figs 2–3). Datasets for the two different species complexes were analyzed separately. The presence or absence of introns varies between these species complexes (Zipfel et al. 2006), and it is therefore not possible to align them in the same dataset. Comparison of the β-tubulin sequence of the isolates in the O. ulmi complex with sequences from GenBank confirmed their identity as O. quercus (Fig. 2). Analysis of the β-tubulin data for the isolates residing in the S. schenckii–O. stenoceras complex showed that the isolates from this study were closely related to Ophiostoma abietinum Marm. & Butin, O. fusiforme and Ophiostoma lunatum Aghayeva & M.J. Wingf. (Fig. 3). These species represent a poorly resolved species complex and the identity of the isolates could not be resolved with certainty but based on morphology and DNA sequence data, it is treated here as O. fusiforme-like. Based on both ITS and β-tubulin data, the third species collected in this study was tentatively identified as O. grandicarpum (Figs 1 and 3) but the fungus was clearly distinct from all other species currently known. It also did not reside in any of the major lineages in Ophiostoma sensu lato (de Beer and Wingfield 2013). Therefore, the taxonomic placement of the isolate could not be further resolved in this study.
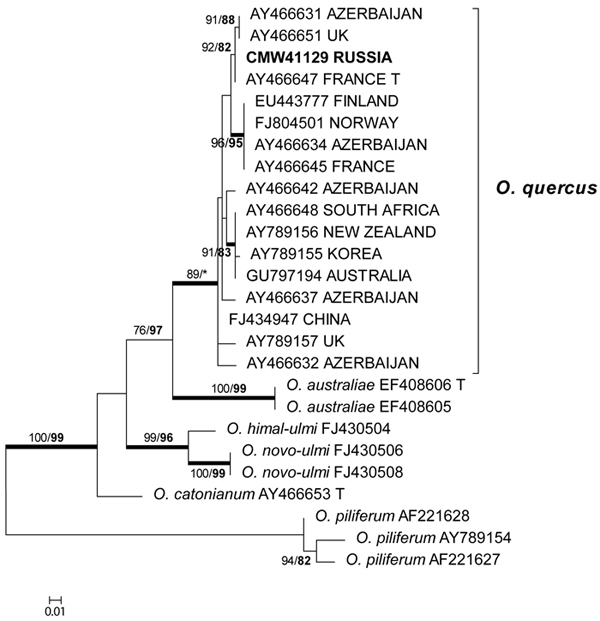
Fig. 2. Phylogram obtained from ML analyses of the β-tubulin gene of species in the O. ulmi complex. Isolate numbers of sequences obtained in this study are printed in bold type. The bootstrap support values for ML (normal type) and MP (bold type) above 70% are indicated at the nodes. Posterior probabilities (above 90%) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 70%. T = ex-type isolates. Scale bar = total nucleotide difference between taxa.
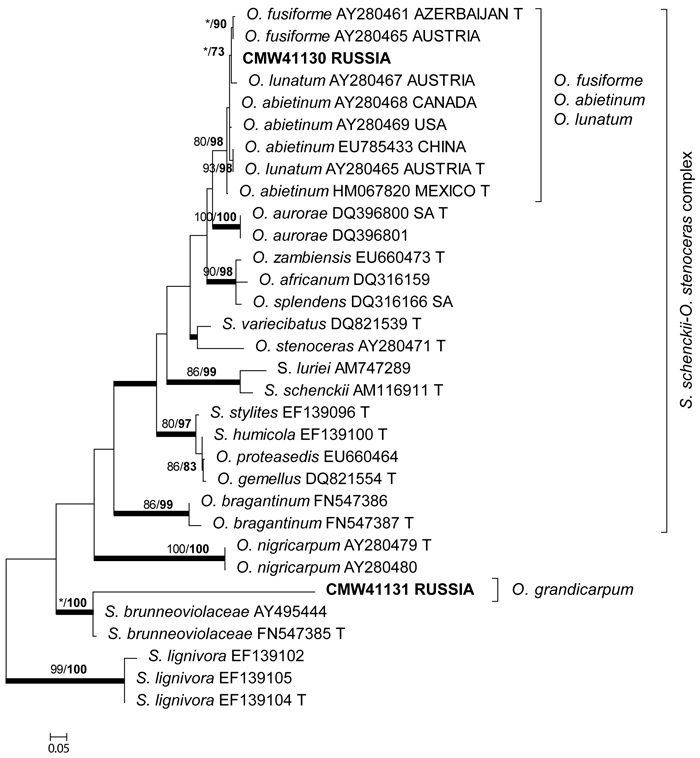
Fig. 3. Phylogram obtained from ML analyses of the β-tubulin gene of species in the S. schenckii–O. stenoceras complex. Isolate numbers of sequences obtained in this study are printed in bold type. The bootstrap support values for ML (normal type) and MP (bold type) above 70% are indicated at the nodes. Posterior probabilities (above 90%) obtained from BI are indicated by bold lines at the relevant branching points. * = bootstrap values lower than 70%. T = ex-type isolates. Scale bar = total nucleotide difference between taxa.
3.3 Pathogenicity tests
Approximately 10 months after the inoculation had commenced, lesion development on phloem of Q. robur trees was assessed (Fig. 4). The agar block inoculation results showed that O. quercus was able to spread in the phloem of young oaks and cause obvious lesions that were significantly larger than those of the controls (Table 3). The fungus identified as O. fusiforme-like caused smaller areas of phloem necrosis. Although the average length of the lesions reached 30 mm, the critical threshold value (≤ 0.05) for statistical significance was not reached and these results failed to confirm a difference from controls. Inoculation with the strain tentatively identified as O. grandicarpum gave rise to the smallest lesions. None of the inoculated fungi caused staining of the sapwood and no external symptoms of oak-decline were seen on the inoculated trees. All species were successfully re-isolated from the lesions. None of the test fungi was isolated from the control inoculations.

Fig. 4. Brown necrosis caused by Ophiostoma quercus in the inner bark of young Quercus robur tree 10 months after the inoculation. Аgar blocks overgrown with the fungus mycelium served an inoculum. Necrosis size: length 47 mm; width 10 mm.
| Table 3. Lesion sizes (x ± m, mm) in the phloem at the end of the inoculation experiment of young Quercus robur trees (size of the wound area is deducted from necrosis area). | |||
| Inoculation variant used in August 2010 | Measured in September 2010 | Measured in May 2011 | |
| Lenght | Lenght | Width | |
| Ophiostoma grandicarpum | 1.7 ± 1.7 | 16.7 ± 8.8 | 0 |
| O. fusiforme-like | 10.3 ± 7.5 | 30.6 ± 8.5 | 4.1 ± 4.1 |
| O. quercus | 5.1 ± 2.3 | 69.1 ± 20.5* | 11.1 ± 3.9 |
| Sterile agar block | 5.7 ± 4.7 | 9.0 ± 3.8 | 0 |
| Control (wounding without inoculation) | 0 | 3.3 ± 3.3 | 3.3 ± 3.3 |
| *Significantly different from control (wounding without inoculation) according to Mann-Whitney test (P ≤ 0.05). | |||
4 Discussion
Results of this study revealed the presence of Ophiostoma spp. on declining Q. robur in Tellerman forest, Russia. These included O. grandicarpum, O. fusiforme-like, and most commonly O. quercus. Previous studies have also reported the common occurrence of species of Ophiostoma from Quercus sp. in Russia (Sczerbin-Parfenenko 1953; Potlaychuk 1957; Potlaychuk and Shekunova 1985; Oleksyn and Przybil 1987; Osipov and Selochnik 1989). These fungi were suggested to be the causal agents of the oak die-back in earlier studies. Reports of these fungi have always been inconsistent and their role as primary pathogens has been rejected (Oleksyn and Przybil 1987; Cech et al. 1990; Simonin et al. 1993; Delatour et al. 1994). Our results lead to a similar conclusion.
The common occurrence of O. quercus on Q. robur trees in this study was not surprising. The fungus was originally described from the same host tree by Georgévitch (1926) from Serbia. Ophiostoma quercus is a common sap stain fungus on hardwoods and it is known to have a worldwide distribution (e.g. Kowalski 1996; de Beer et al. 2003; Geldenhuis et al. 2004; Kamgan Nkuekam et al. 2008; Linnakoski et al. 2008, 2009; Grobbelaar et al. 2009; Paciura et al 2010). The species is morphologically and genetically highly diverse (Brasier 1993; Przybyl and Morelet 1993; Grobbelaar et al. 2009). It is likely that prior to the availability of DNA-based identification methods, various related species were attributed to this name or fungi that are now regarded as synonyms of the species. For example, in the former USSR literature, the causal agents of oak decline were reported to be O. quercus, O. roboris, O. valachicum and O. kubanicum (Oleksyn and Przybil 1987). Ophiostoma roboris has been shown to be a synonym to O. quercus (Grobbelaar et al. 2009; de Beer et al. 2013), but the taxomic status of the latter two oak-associated species remains unclear. Ophiostoma valachicum has been treated as nomium dubium (Upadhyay 1981) or a synonym to O. piceae (Przybyl and de Hoog 1989) and O. quercus (Harrington et al. 2001). Based on the morphological characteristics as described in the original description (Georgescu et al. 1948), O. valachicum is currently recognized as a valid species that is distinct from species in the O. piceae complex (Grobbelaar et al. 2009; de Beer et al. 2013). However, authentic material does not exist and neotypification of the species is necessary to resolve its taxonomic placement. Ophiostoma kubanicum is another species originally described from oak (Sczerbin-Parfenenko 1953), but excluded from the genus Ophiostoma because it was invalidly published and a lack of authentic material does not allow its validation (Grobbelaar et al. 2009; de Beer et al. 2013).
This study represents the first record of O. grandicarpum from Russia. Ophiostoma grandicarpum is a rarely encountered fungus, which is characterized by extraordinarily long ascomatal necks (Kowalski and Butin 1989). The fungus has previously been reported only from Q. robur from Poland, Czech Republic and Germany (Kowalski and Butin 1989; Kowalski 1991; Kehr and Wulf 1993; Čížková et al. 2005; Novotný and Šrůtka 2004) and its presence in Russia is thus not surprising. Only one reference sequence is currently available in GenBank, and it does not represent the type strain (CBS 250.88) from Poland (Kowalski and Butin 1989). Our results based on ITS and β-tubulin sequence data support the previous views that this fungus is of uncertain generic affiliation in the Ophiostomatales (Aghayeva et al. 2004; de Beer and Wingfield 2013).
The fungus identified in this study as O. fusiforme-like, represents a species that resides in the S. schenckii–O. stenoceras complex and grouped together with O. fusiforme, O. lunatum and O. abietinum. Although these fungi have been described as distinct taxa (Marmolejo and Butin 1990; Aghayeva et al. 2004), several recent phylogenetic studies have shown that the three species group together (Min et al. 2009; Matsuda et al. 2010; Linnakoski et al. 2010; de Beer and Wingfield 2013). Clearly the fungus emerging from the present study requires further investigation.
The preliminary inoculation test on Q. robur showed that only one of the test fungi, O. quercus, caused lesions statistically different to those of the controls. However, no external symptoms of die-back were observed on inoculated Q. robur trees. Our results are consistent with previous studies that have reported O. quercus to result in lesions when artificially inoculated on Q. robur trees, but without symptoms of die-back (Simonin et al. 1993; Delatour et al. 1994). The other ophiostomatoid species (O. fusiforme-like and O. grandicarpum) caused small lesions, suggesting that the species are non-pathogenic. Since a relatively small number of trees were used in this study, it is not possible to derive comprehensive conclusions regarding pathogenicity of the strains tested. However, our preliminary results suggest that fungi investigated in this study are unlikely to play causal role in oak die-back. Based on current knowledge, these fungi are considered as a saprotrophic species that may exist as endophytes on Q. robur (Selochnik 2002). Clearly, more detailed investigations are needed to clarify to potential endophytic existence of ophiostomatoid fungi. Our results are in agreement with the previous studies that have concluded that ophiostomatoid fungi are most likely only components of a complex syndrome that involves of abiotic, biotic and anthropogenic factors that have contributed to a die-back of Quercus spp. in Russia as well as other parts of Europe (Cech et al. 1990; Ciesla and Donaubauer 1994; Führer 1998).
The results of this study provide a foundation to estimate the biodiversity of ophiostomatoid fungi associated with Q. robur in Russia. The new records of species encountered, covering only a small geographic area, indicate that the biodiversity these fungi remains incompletely understood. The study also provided preliminary data regarding the pathogenic potential of the fungal species found on Quercus spp. in Russia. Although these fungi seem to not to be pathogenic in their native range, there is a risk of their being accidentally introduced into new areas via global trade, where native hosts in new environments could respond negatively.
Acknowledgements
We thank Dr. Wilhelm de Beer from the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, for his advice regarding the phylogenetic component of this study. The study was financially supported by the University of Helsinki and the Emil Aaltonen Foundation, Finland; the members of the Tree Protection Co-operative Programme (TPCP), the THRIP initiative of the Department of Trade and Industry, and the University of Pretoria, South Africa.
References
Aghayeva D.N., Wingfield M.J., de Beer Z.W., Kirisits T. (2004). Two new Ophiostoma species with Sporothrix anamorphs from Austria and Azerbaijan. Mycologia 96: 866–878. http://dx.doi.org/10.2307/3762119.
Brasier C.M. (1993) The genetic system as a fungal taxonomy tool: gene flow, molecular variation and sibling species in the ‘Ophiostoma piceae – Ophiostoma ulmi’ complex and its taxonomic and ecological significance. In: Wingfield M.J., Seifert K.A., Webber J.F. (eds.). Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St Paul, MN. p. 77–92.
Cech T., Donoubauer E., Tomiczek C., Leontovyc R., Yde-Anderson A., Delatour C., Wulf A., Toth J., Vajna L., Vammini A., Luisi N., Oosterbaan A., Kowalski T., Gibbs J.N., Jurc D. (1990). Oak decline and status of Ophiostoma spp. on oak in Europe. Fungi associated with oak decline. OEPP/EPPO Bulletin 20: 405–422. http://dx.doi.org/10.1111/j.1365-2338.1990.tb00164.x.
Ciesla W.M., Donaubauer E. (1994). Decline and dieback of trees and forests. A global overview. FAO Forestry Paper 120. 92 p.
Čížková D., Šrůtka P., Kolařík M., Kubátová A., Pažoutová P. (2005). Assessing the pathogenic effect of Fusarium, Geosmithia and Ophiostoma fungi from broad-leaved trees. Folia Microbiologica 50: 59–62. http://dx.doi.org/10.1007/BF02931294.
de Beer Z.W., Wingfield M.J. (2013). Emerging lineages in the Ophiostomatales. In: Seifert K.A., de Beer Z.W, Wingfield M.J. (eds.). Ophiostomatoid fungi: expanding frontiers. Utrecht, CBS-KNAW Fungal Biodiversity Centre. CBS Biodiversity Series 12: 21–46.
de Beer Z.W., Wingfield B.D., Wingfield M.J. (2003). The Ophiostoma piceae- complex in the southern hemisphere: a phylogenetic study. Mycological Research 107: 469–476. http://dx.doi.org/10.1017/S0953756203007445.
de Beer Z.W., Seifert K.A., Wingfield M.J. (2013). A nomenclator for ophiostomatoid genera and species in the Ophiostomatales and Microascales. In: Seifert K.A., de Beer Z.W, Wingfield M.J. (eds.). Ophiostomatoid fungi: Expanding frontiers. Utrecht, CBS-KNAW Fungal Biodiversity Centre. CBS Biodiversity Series 12: 261–268.
Delatour C., Morelet M., Ménard J.-E. (1994). Ophiostomas, a possible cause of oak dieback. Ravue Forestiere Francaise 46: 446–452. [In French].
Führer E. (1998). Oak decline in Central Europe: a synopsis of hypothesis. In: McManus M.L., Liebhold A.M. (eds.). Proceedings of population dynamics, impacts, and integrated management of forest defoliating insects. USDA Forest Service General Technical Report NE-247. p. 7–24.
Gardes M., Bruns T.D. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118.
Geldenhuis M.M., Roux J., Montenegro F., de Beer Z.W., Wingfield M.J., Wingfield B.D. (2004). Identification and pathogenicity of Graphium and Pesotum species from machete wounds on Schizolobium parahybum in Ecuador. Fungal Diversity 15: 135–149.
Georgevitch P. (1926). Ceratostomella querci n. sp. Comptes Rendus Acedémie des Sciences 183: 759–761.
Georgevitch P. (1927). Ceratostomella quercus n. sp. Ein parasit der Slavonischen Eichen. Biologia Generalis 3: 245–252.
Georgescu C., Teodoru I., Badea M. (1948). Uscarea in masa a Stejarului extras. Analele Institutul Cercetări Forestiere Al Românie (Bucuresti) 11: 185–223.
Glass N.L., Donaldson G.C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 6: 1323–1330.
Grobbelaar J.W., Aghayeva D.N., de Beer Z.W., Bloomer P., Wingfield M.J., Wingfield B.D. (2009). Delimitation of Ophiostoma quercus and its synonyms using multiple gene phylogenies. Mycological Progress 8: 221–226. http://dx.doi.org/10.1007/s11557-009-0594-4.
Guseinov E.S. (1984). Vascular dieback of oak in Azerbaijan. Mycology and Phytopathology 18: 144–149. [In Russian].
Harrington T.C., McNew D., Steimel J., Hofstra D., Farrell R. (2001). Phylogeny and taxonomy of the Ophiostoma piceae complex and the Dutch Elm Disease fungi. Mycologia 93: 111–136. http://dx.doi.org/10.2307/3761610.
Ivanchenko Y.N. (1957). The causes of oak wilt in the Lipetsky garde nof the vSavalskiy leshoz. Trudy Vsesoyuznogo Instituta Zashchity Rasteniy (Proceedings of the All-Union Institute of Plant Protection) 8: 227–237. [In Russian].
Kamgan Nkuekam G., Jacobs K., de Beer Z.W., Wingfield M.J., Roux J. (2008). Pesotum australi sp. nov. and Ophiostoma quercus associated with Acacia mearnsii trees in Australia and Uganda, respectively. Australasian Plant Pathology 37: 406–416. http://dx.doi.org/10.1071/AP08027.
Katoh K., Standley D.M. (2013). MAFFT multiple sequence aligment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. http://dx.doi.org/10.1093/molbev/mst010.
Kehr R.D., Wulf A. (1993). Fungi associated with above-ground portions of declining oaks (Quercus robur) in Germany. European Journal of Forest Pathology 23: 18–27.
Kowalski T. (1991). Oak decline I. Fungi associated with various disease symptoms on overground portions of middleaged and old oak (Quercus robur L.). European Journal of Forest Pathology 21: 136–151.
Kowalski T. (1996). Oak decline II. Fungi associated with various types of lesions on stems and branches of young oaks (Quercus robur). Österreichische Zeitschrift für Pilzkunde 5: 51–63.
Kowalski T., Butin H. (1989). Taxonomie bekannter and neuer Ceratocystis – Arten and Eiche (Quercus robur L.). Journal of Phytopathology 124: 236–238.
Kryukova E.A., Balder H. (1993). The problem of infectious dieback of oak. Lesnoe Khozuaistvo 6: 46–48. [In Russian].
Kuzmichev E.P. (1983). Vascular mycosis of oak, its distribution and ecology in the southeast of the European part of the RSFSR. Abstract of PhD thesis in biology. Ural Forest Institute, Sverdlovsk. 19 p. [In Russian].
Linnakoski R., de Beer Z.W., Rousi M., Niemelä P., Pappinen A., Wingfield M.J. (2008). Fungi, including Ophiostoma karelicum sp. nov., associated with Scolytus ratzeburgi infesting birch in Finland and Russia. Mycological Research 112: 1475–1488. http://dx.doi.org/10.1016/j.mycres.2008.06.007.
Linnakoski R., de Beer Z.W., Rousi M., Solheim H., Wingfield M.J. (2009). Ophiostoma denticiliatum sp. nov. and other Ophiostoma species associated with the birch bark beetle in southern Norway. Persoonia 23: 9–15. http://dx.doi.org/10.3767/003158509X468038.
Linnakoski R., de Beer Z.W., Ahtiainen J., Sidorov E., Niemelä P., Pappinen A., Wingfield M.J. (2010). Ophiostoma spp. associated with pine- and spruce-infesting bark beetles in Finland and Russia. Persoonia 25: 72–93. http://dx.doi.org/10.3767/003158510X550845.
Marmolejo J.G., Butin H. (1990). New conifer-inhabiting species of Ophiostoma and Ceratocystiopsis (Ascomycetes, Microascales) from Mexico. Sydowia 42: 193–199.
Matsuda Y., Kimura K., Ito S. (2010). Genetic characterization of Raffaelea quercivora isolates collected from areas of oak wilt in Japan. Mycoscience 51: 310–316. http://dx.doi.org/10.1007/s10267-010-0040-0.
Miller M.A., Pfeiffer W., Schwartz T. (2010). Creating the CIPRES Sciences Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments workshop (GCE). New Orleans, LA, USA. November 14, 2010. p. 1–8. http://dx.doi.org/10.1109/GCE.2010.5676129.
Min L., Zhou X.D., de Beer Z.W., Wingfield M.J., Sun J-H. (2009). Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Diversity 38: 133–145.
Minkevich I.I. (1962). Vascular disease of oak. Lesnoe Khozuaistvo 15(10): 48.
Minkevich I.I. (1964). Specialization and variability of vascular mycosis agents on tree species. Botanical Journal 49: 6.
Novotný D., Šrůtka P. (2004). Ophiostoma stenoceras and O. grandicarpum (Ophiostomatales) first records in the Czech Republic. Czech Mycology 56: 19–32.
Nylander J.A.A. (2004). MrModeltest v.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden.
O’Donnel K., Cigelnik E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. http://dx.doi.org/10.1006/mpev.1996.0376.
OEPP/EPPO (1983). Data sheet on quarantine organism no. 76: Ophiostoma roboris. EPPO Bulletin 13: 11–31.
Oleksyn J., Przybil K. (1987). Oak decline in the Soviet Union – scale and hypotheses. European Journal of Forest Pathology 6: 321–336.
Osipov V.V., Selochnik N.N. (1989). The state of oak forests in the Central Russian forest-steppe according to reconnoitering inspections in 1984–1987. In: Osipov V.V., Selochnik N.N., Ilyushenko A.F. (eds.). The state of oak forests in the forest-steppe zone. Publishing House “Nauka”, Moscow. p. 199–205. [In Russian].
Osipov V.V., Selochnik N.N., Ilyushenko A.F. et al. (1989). The state of oak forests in the forest-steppe zone. Publishing House “Nauka”, Moscow. 230 p. [In Russian].
Paciura D., Zhou X.D., De Beer Z.W., Jacobs K., Ye H., Wingfield M.J. (2010). Characterisation of synnematous bark beetle-associated fungi from China, including Graphium carbonarium sp. nov. Fungal Diversity 40: 75–88. http://dx.doi.org/10.1007/s13225-009-0004-x.
Pattengale N.D., Alipour M., Bininda-Emonds O.R., Moret B.M., Stamakis A. (2010). How many bootstrap replicates are necessary? Journal of Computational Biology 17: 337–354. http://dx.doi.org/10.1089/cmb.2009.0179.
Petrescu M. (1974). Le deperissement du chene in Roumanie. European Journal of Forest Pathology 4: 222–227. http://dx.doi.org/10.1111/j.1439-0329.1974.tb00440.x. [In French].
Potlaychuk V.I. (1957). On the biology of the causal agent of oak wilt. Trudy Vsesoyuznogo Instituta Zashchity Rasteniy (Proceedings of the All-Union Institute of Plant Protection) 8: 227–237. [In Russian].
Potlaychuk V.I., Shekunova E.G. (1985). Distribution of Ceratocystis Ell. et Halst. Emend Bakschi species in USSR. Novosti Sistematiki Nizshikh Rasteniy (The News of Lower Plant Systematic) 22: 148–156. [In Russian].
Przybyl K., de Hoog G.S. (1989). On the variability of Ophiostoma piceae. Antonie van Leeuwenhoek 2: 177–178.
Przybyl K., Morelet M. (1993). Morphological differences between Ophiostoma piceae and O. querci, and among O. querci isolates. Cryptogamie Mycologie 14: 219–228.
Ronquist F., Huelsenbeck J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. http://dx.doi.org/10.1093/bioinformatics/btg180.
Sczerbin-Parfenenko A.L. (1953). Cancerous and vascular diseases of decidious trees. Goslesbumizdat Publ, Moscow-Leningrad. 90 p.
Selochnik N.N. (1998). Tracheomycosis of oak. Mycology and Phytopathology 4: 63–74. [In Russian].
Selochnik N.N. (2000). Tracheomycosis in oak forests of the Russian forest-steppe. In: Osipov V.V., Selochnik N.N., Ilyushenko A.F. (eds.). Fungal communities of forest ecosystems. Karelsky Research Centre of RAS, Moscow-Petrozavodsk. p. 207–223. [In Russian].
Selochnik N.N. (2002). Behavior of ophiostomatoid fungi in oak: from parasitism to the endophytic existence. In: Proceedings of First National Congress of Mycologists of Russia. Synapsis Reports. Moscow. p. 205–206. [In Russian].
Selochnik N.N., Kondrashova N.K. (1989). Vascular mycosis of oak. Diagnosis of the disease and identification of the agent. In: Osipov V.V., Selochnik N.N., Ilyushenko A.F., et al. (eds.). The state of oak forests in the forest-steppe zone. Publishing House “Nauka”, Moscow. p. 171–180. [In Russian].
Selochnik N.N., Pashenova N.V. (2007). Ophiostoma fungi on common oak (Quercus robur L.) in Russia. In: Kovalenko A., Melnik V., Vedenyapina E., Zmitrovich I. (eds.). Proceedings of XV International Congress of European Mycologists. September, 16–21, 2007. Ed. by St. Petersburg: TREEART LLC. p. 57–58. [In Russian].
Simonin G., Cochard H., Delatour C., Granier A., Dreyer E. (1993). Vulnerability of young oak seedlings (Quercus robur L) to embolism: responses to drought and to an inoculation with Ophiostoma querci (Georgevitch) Nannf. Annals of Forest Science 51: 493–504. http://dx.doi.org/10.1051/forest:19940505.
Stamakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. http://dx.doi.org/10.1093/bioinformatics/btu033.
Stamakis A., Hoover P., Rougemont J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. http://dx.doi.org/10.1080/10635150802429642.
Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. http://dx.doi.org/10.1093/molbev/mst197.
Upadhyay H. (1981). A monograph of Ceratocystis and Ceratocystiopsis. University of Georgia Press, Athens, GA, USA. 176 p.
White T.J., Bruns T., Lee S., Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J. (eds.). PCR protocols: a guide to methods and applications. Academic Press, San Diego, California. p. 315–321.
Wingfield M.J., Seifert K.A., Webber J.F. (eds.) (1993). Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St Paul, MN. 293 p.
Zipfel R.D., de Beer Z.W, Jacobs K., Wingfield B.D., Wingfield M.J. (2006). Multi- gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Studies in Mycology 55: 75–97.
Total of 66 references.

