Biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China: a comprehensive inventory of a 2 ha plot
Liu L.-B., Wu Y.-Y., Hu G., Zhang Z.-H., Cheng A.-Y., Wang S.-J., Ni J. (2016). Biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China: a comprehensive inventory of a 2 ha plot. Silva Fennica vol. 50 no. 3 article id 1492. https://doi.org/10.14214/sf.1492
Highlights
- Comprehensive inventory of the karst secondary forest based on a 2 ha large plot enhanced the reliability of biomass estimates
- The biomass was 158.1 Mg ha−1, and the five dominant tree species accounted for 92.4% of aboveground tree biomass
- The estimated necromass of woody debris and litter in the karst secondary forest was 17.6 Mg ha−1.
Abstract
The biomass of a secondary evergreen and deciduous broad-leaved mixed forest was comprehensively inventoried in a permanent 2 ha plot in southwestern China. Biomass models, sub-sampling, soil pit method, and published data were utilized to determine the biomass of all components. Results showed that the total biomass of the forest was 158.1 Mg ha−1; the total biomass included the major aboveground (137.7 Mg ha−1) and belowground (20.3 Mg ha−1) biomass components of vascular plants as well as the minor biomass components of bryophytes (0.078 Mg ha−1) and lichens (0.043 Mg ha−1). The necromass was 17.6 Mg ha−1 and included woody debris (9.0 Mg ha−1) and litter (8.6 Mg ha−1). The spatial pattern of the aboveground biomass was determined by the spatial distribution of dominant trees with large diameter, tall height, and dense wood. The belowground biomass differed in terms of root diameter and decreased with increasing soil depth. The belowground biomass in each soil pit in local habitats was not related to the spatial distribution of woody plants and soil pit depth. The karst forest presented lower biomass compared than the nonkarst forests in the subtropical zone. Biomass carbon in the karst terrains would increase substantially if degraded karst vegetation could be successfully restored to the forest. Comprehensive site-based biomass inventory of karst vegetation will contribute not only to provide data for benchmarking global and regional vegetation and carbon models but also for regional carbon inventory and vegetation restoration.
Keywords
biomass;
secondary forest;
necromass;
karst terrain;
carbon inventory
- Liu, State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Lincheng West Road 99, 550081 Guiyang, China; Puding Karst Ecosystem Research Station, Chinese Academy of Sciences, 562100 Puding, China; University of Chinese Academy of Sciences, 100049 Beijing, China E-mail liulibin@mail.gyig.ac.cn
- Wu, State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Lincheng West Road 99, 550081 Guiyang, China; Puding Karst Ecosystem Research Station, Chinese Academy of Sciences, 562100 Puding, China; University of Chinese Academy of Sciences, 100049 Beijing, China E-mail wuyang2468@hotmail.com
- Hu, School of Chemistry and Life Science, Guangxi Teachers Education University, 530001 Nanning, China; Key Laboratory of Beibu Gulf Environment Change and Resources Utilization of Ministry of Education, Guangxi Teachers Education University, 530001 Nanning, China E-mail ahhugang@gmail.com
- Zhang, School of Chemistry and Life Science, Guangxi Teachers Education University, 530001 Nanning, China; Key Laboratory of Beibu Gulf Environment Change and Resources Utilization of Ministry of Education, Guangxi Teachers Education University, 530001 Nanning, China E-mail gxtczzh@gmail.com
- Cheng, State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Lincheng West Road 99, 550081 Guiyang, China; Puding Karst Ecosystem Research Station, Chinese Academy of Sciences, 562100 Puding, China E-mail chenganyun@vip.skleg.cn
- Wang, State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Lincheng West Road 99, 550081 Guiyang, China; Puding Karst Ecosystem Research Station, Chinese Academy of Sciences, 562100 Puding, China E-mail wangshijie@vip.skleg.cn
-
Ni,
State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Lincheng West Road 99, 550081 Guiyang, China; Puding Karst Ecosystem Research Station, Chinese Academy of Sciences, 562100 Puding, China
E-mail
nijian@vip.skleg.cn

Received 11 September 2015 Accepted 14 June 2016 Published 20 June 2016
Views 120896
Available at https://doi.org/10.14214/sf.1492 | Download PDF

1 Introduction
Biomass is a key to understand terrestrial carbon storage and carbon cycle from the local to the regional and global scales. Various methods, such as forest inventory, remote sensing, and vegetation modeling, are used to comprehensively estimate vegetation biomass along with the development of global studies from the mid 1980s and the emergence of regional to global carbon inventory (Melillo et al. 1993; Dixon et al. 1994; Ni et al. 2001, 2003; Zhou et al. 2002; Powell et al. 2010). Important and accurate techniques are available to comprehensively estimate site-based biomass in any vegetation area; these methods include biomass predictions based on direct field biomass measurements, such as estimates of aboveground biomass (AGB) and belowground biomass (BGB) through sub-sampling, biomass model, and soil pit methods. These techniques were promoted by the Man and the Biosphere Program and the International Biological Program in the 1960s–1970s. These estimation methods are important to extend local carbon inventory to regional and global scales, validate remote-sensing biomass functions, and parameterize vegetation and carbon models (Luyssaert et al. 2007).
Forest is the largest vegetated area globally and the largest carbon pool in the global terrestrial carbon budget (Dixon et al. 1994; Schimel et al. 2001). The biomass of a forest has been intensively inventoried using different methods at various spatial and temporal scales (Lieth and Whittaker 1975; Feng et al. 1999; Luyssaert et al. 2007). However, biomass characteristics of some types of forest in remote or certain topographical areas have not been determined yet. For example, mixed evergreen and deciduous broad-leaved forests with a large proportion of deciduous woody plants grow in the karst landscape in tropical and subtropical regions. With its long-term chemical weathering and denudation, karst landscape is known for its high rate of outcrops, shallow soils, water leakage, and heterogeneity of habitats. Karst forests often grow on rocks or in fissures, thereby complicating biomass sampling, especially for roots. Consequently, few biomass studies on karst forests have been conducted compared with studies on nonkarst forests.
Southwestern China – especially the subtropical Guizhou province, Guangxi autonomous region, and Yunnan province – is one of the three major areas worldwide with vast distribution of karst landscape. Biomass models and sub-sampling method have been utilized to estimate AGB of southern subtropical evergreen and deciduous broad-leaved mixed forests in a peak-clump depression karst terrain in southern Guizhou. The results showed that the AGB of this old-growth karst forest was significantly lower than that of other typical evergreen broad-leaved forests at similar latitudes (Yang and Cheng 1991; Zhu et al. 1995; Yu et al. 2002). Belowground biomass of different vegetation restoration stages at the same site has also been estimated using specific sampling method of digging four soil pits around an averaged sample tree (Luo et al. 2010), as well as through normal soil pit method with 10 soil pits in a plot (Ni et al. 2015). These sampling methods revealed higher BGB and root–shoot ratio of the karst forest than that of typical evergreen broad-leaved forests in subtropical China. The total biomass of a forest in a peak-forest plain karst in northwestern Guangxi was considerably lower than that of the karst forest biomass mentioned above. The biomass sharply decreased over time because of human disturbances (Zeng et al. 2007). Moreover, biomass models were applied to estimate AGB of plateau-surface-type karst secondary forests in central Guizhou (Liu et al. 2009; Liu et al. 2013). The results showed that these forests possessed lower AGB than that of the peak-clump depression karst forests. The total biomass of karst shrub communities in the same area and surrounding regions was measured using sub-sampling (Tu and Yang 1995). The measured biomass was lower than that of nonkarst shrub in the same bioclimatic zone. The total biomass of various types of vegetation in a trough-valley-type karst area in northern Guizhou was further estimated using biomass models and sub-sampling method (Zhong et al. 2014); the results indicated lower biomass than those of the peak-clump depression-type and plateau-surface-type karst forests. Low ABG and high BGB of karst vegetation could be due to harsh habitats, which constrained the growth of green biomass (Zhu et al. 1995) and led to resource allocation to the roots (Ni et al. 2015).
Most biomass estimation studies focused on AGB (Yang and Cheng 1991; Zhu et al. 1995; Yu et al. 2002; Liu et al. 2009), whereas BGB estimates have been rarely reported (Tu and Yang 1995; Zeng et al. 2007; Luo et al. 2010; Ni et al. 2015). Belowground biomass estimates were also accompanied with significant uncertainties caused by small sample size in soil pit methods. Furthermore, the biomass of bryophytes and lichens as well as the necromass of woody debris have not been estimated yet. Previous studies utilized field estimates of small plots, ranging from 400 m2 to 900 m2, which could not represent the entire feature of the considerably heterogeneous karst habitats. Biomass estimates based on large plots and high number of belowground samples should be performed.
Karst terrains occupy a large area of ca. 22 000 000 km2 worldwide, accounting for approximately 14.8% of the global land area (Jiang et al. 2014). Karst landscape in southern China covers 510 000 km2, which is approximately 5.8% of the total land area of China (Jiang et al. 2014). Karst vegetation is seriously degraded to shrublands and tussocks because of human activities and intensive land use, resulting in severe rocky desertification, which is a desert-like landscape with exposed rocks. Shrubland vegetation currently occupies 23.4% of the total vegetation area in whole southwestern China (ECVMC 2007). Natural and even secondary forests are only distributed in several small areas. Karst regions have low biomass, and forest recovery from shrubland exhibits potential to enhance carbon storage (Yang and Chen, 1991; Ni et al. 2015). Studies on site-based biomass inventory of karst forest in this region should be conducted to precisely estimate the potential of carbon storage in the karst landscape and elucidate the regional carbon budget.
The present study area is a 2 ha permanent plot in a secondary evergreen and deciduous broad-leaved mixed forest in central Guizhou Plateau, southwestern China. All components of biomass (AGB, BGB, bryophytes, and lichens) and necromass (woody debris and litter) were inventoried. The AGB spatial pattern of the forest was also analyzed. This study will provide basic data to conduct a complete inventory of the regional vegetation biomass and carbon storage of rocky desertification areas in karst regions in southern China.
2 Materials and methods
2.1 Study area
Plateau-surface karst morphology is one of the eight karst landscapes in southern China and is mainly distributed in the central Guizhou Plateau. Puding county, located in the watershed between the Yangtze and Pearl Rivers, is one of the representative areas of the region, especially its Houzhaihe River catchment with an area of 81 km2. This area is located in the middle subtropical China and controlled by monsoon climates. According to records from the Puding meteorological station from 1961 to 2008, the mean annual temperature of the region was 15.1 °C, with temperatures of 5.4 °C and 22.9 °C in January and July, respectively. The average annual precipitation was 1367 mm, of which more than 70% occurred from May to September. The area was cloudy with an annual average sunshine of 1177 h. The altitude of the Houzhaihe River catchment is between 1100 and 1400 m above sea level, with a relative height of most hills at 100–200 m. Limestone and dolomite, particularly the former, are distributed everywhere in the catchment. Secondary evergreen and deciduous broad-leaved mixed forests are distributed in hill tops with less human disturbances. Degraded shrublands, tussocks, and grasslands are found at the middle and foot of hills. Rice fields are the key land use type in the drainage basin area.
A 2 ha (horizontally-projected area) permanent monitoring plot (Fig. 1) at the Tianlongshan site (26°14´N, 105°45´E, 1402–1512 m a.s.l.) represents the vegetation, soil, and karst morphology of the whole Houzhaihe River catchment; this plot was established in 2012 after a complete vegetation survey. This plot almost occupied the entire southern slope of a small hill with a relative altitudinal range of 110 m. The plot was large enough to cover all typical karst vegetation types and soils. The slope was very steep, with an average grade of 31.0° ± 14.0° (visually estimated based on 200 quadrats) and could reach 80°. Limestone outcrops were distributed everywhere, with an average coverage of 44.7% ± 25.8% and reached 98% in several areas. Brown limestone soil, known as “Rendzina” in the FAO soil classification system, was dominant with a shallow depth of 10–80 cm but with relatively rich soil nutrient.
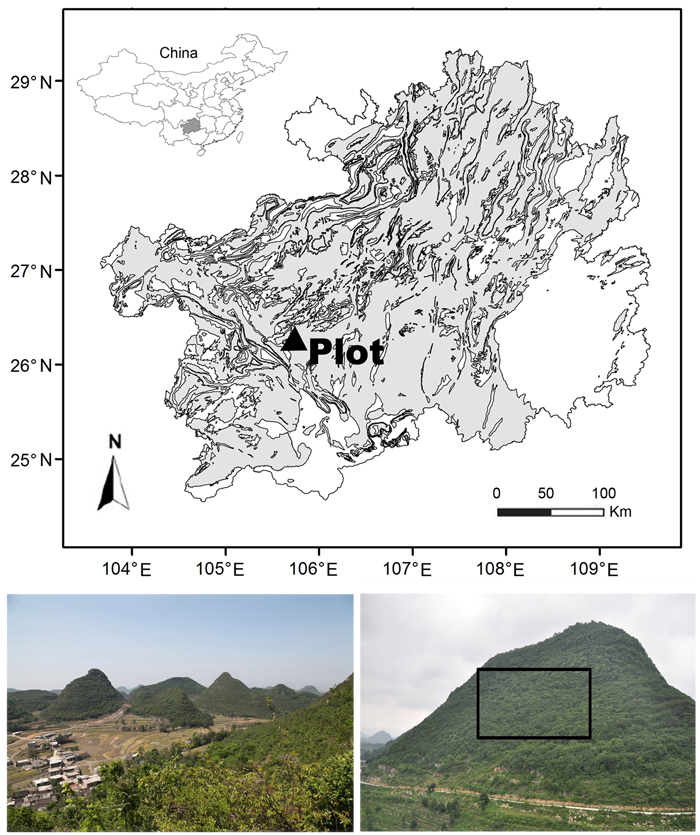
Fig. 1. Location and physiognomy of the Houzhaihe River catchment (left bottom) and the Tianlongshan permanent monitoring plot (right bottom) in the distribution map of karst terrain (the grey) in Guizhou province, southwestern China.
In this typical karst habitat, the evergreen and deciduous broad-leaved mixed forest that grew secondarily from a forest cutting in late 1950s was in the succession stage of subclimax. The height of the tree layer was 6–10 m, with an average diameter at breast height (DBH) of 5.4 ± 4.3 cm and a coverage of 73.1% ± 10.1%. Lithocarpus confinis Huang, Platycarya strobilacea Sieb. et Zucc., Itea yunnanensis Franch., Machilus cavaleriei Levl., and Carpinus pubescens Burk. (All botanical nomenclatures were based on Chen 1982–2004) were the five dominant species in the study area. Pittosporum brevicalyx (Oliv.) Gagnep, Lindera communis Hemsl., Celtis sinensis Pers., and Ilex corallina Franch. were also common. The height of the shrub layer was generally lower than 3 m, with a coverage of approximately 30%. Stachyurus obovatus (Rehd.) Hand.-Mazz., Rhamnus heterophylla Schneid, Myrsine africana L., and several Zanthoxylum species were common shrub species. The herb layer was generally lower than 50 cm, with a coverage of approximately 20%. The main species were Arthraxon prionodes (Roxb.) Hochst., Senecio scandens Buch.-Ham. ex D. Don, Woodwardia unigemmata (Makino) Nakai, and several Carex species. Lianas and vines were well developed and occupied important niches in the community. For example, Dalbergia hancei Benth. was ca. 10 cm thick and more than 40 m long.
2.2 Biomass and necromass estimates
2.2.1 AGB of green vascular plants
We inventoried the entire plot during the summer of 2012 in accordance with the field survey protocol of global forest dynamic permanent plots (Condit 1995). The plot was divided into 200 quadrats with an area of 10 m × 10 m, each plot was designated as the basic vegetation survey unit. Habitat information, which included slope, aspect, coverage of outcrops and of vegetation, was recorded. All woody plants within DBH ≥ 1 cm were labeled and located (relative positions). Species (Chen 1982–2004), DBH, height (or length), and crown width were also recorded. A sub-quadrat (2 m × 2 m) was established at the center of each quadrat. Species, height (or length), basal diameter, and coverage of each shrub and liana and vine as well as species, height, and coverage of herbs were recorded.
Liu et al. (2009) and Liu et al. (2013) cut 172 and 136 sample trees, respectively, in Tianlongshan and Zhaojiatian site in the same catchment. Biomass models of seven tree and 12 shrub species were finally obtained and used to estimate AGB of most species in the present plot (Table 1). The biomass models of other tree species that were not mentioned above, and of lianas and vines were obtained from previous studies performed in surrounding regions (Zhu et al. 1995; Deng et al. 2000; Yang et al. 2003; He et al. 2007; Yuan et al. 2009). Aboveground biomass of individual trees, shrubs, and lianas or vines were predicted using their respective biomass models (Table 1). The total AGB of the plot was the sum of the AGBs of individual woody plants and of herbs obtained from sub-sampling method in the field.
| Table 1. Biomass models used for predicting woody species biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China. Species identification was based on Chen (1982–2004). The units of biomass, DBH (or basal diameter), and height (or length) in the studies of Liu et al. (2009), Liu et al. (2013), and Yang et al. (2003) were g, cm and cm, respectively; those in the studies of Zhu et al. (1995), Deng et al. (2000), He et al. (2007), and Yuan et al. (2009) were kg, cm and m, respectively. | ||||
| Species / DBH classes (cm) | Biomass models | Correlation coefficient | References | |
| Trees | Platycarya strobilacea Sieb. et Zucc. | W = 1.9611(D2H)0.8921 | 0.9886 | Liu et al. 2009 |
| Lithocarpus confinis Huang | W = 0.8967(D2H)0.9636 | 0.9817 | Liu et al. 2009 | |
| Itea yunnanensis Franch. | W = 1.9545(D2H)0.8996 | 0.9568 | Liu et al. 2009 | |
| Machilus cavaleriei Levl. | W = 2.6211(D2H)0.8565 | 0.9733 | Liu et al. 2009 | |
| Carpinus pubescens Burk. | Wl = 0.3644(D2H)0.7443 | 0.971 | Liu et al. 2013 | |
| Ww = 0.8076(D2H)0.9378 | 0.998 | Liu et al. 2013 | ||
| Pittosporum brevicalyx (Oliv.) Gagnep | W = 0.0755(D2H)0.8941 | 0.9872 | Zhu et al. 1995 | |
| Lindera communis Hemsl. | W = 0.0755(D2H)0.8941 | 0.9872 | Zhu et al. 1995 | |
| Ilex: Ilex corallina Franch., Ilex macrocarpa Oliv. | W = 0.0755(D2H)0.8941 | 0.9872 | Zhu et al. 1995 | |
| Kalopanax septemlobus (Thunb.) Koidz. | W = 1.1416(D2H)0.8828 | 0.9967 | Liu et al. 2009 | |
| Lauraceae: Cinnamomum bodinieri Levl., Lindera pulcherrima var. hemsleyana, Litsea rubescens Lec. | Wt = 1.728 + 0.015D2H | 0.938 | He et al. 2007 | |
| Wr = 0.379 + 0.002D2H | 0.982 | He et al. 2007 | ||
| Celtis sinensis Pers., llex franchetiana Loes., lbizia kalkora (Roxb.) Prain | Log Ws = 0.75995logD2H – 0.75237 | 0.948 | Deng et al. 2000 | |
| Log Wb = 0.69997logD2H – 0.93934 | 0.959 | Deng et al. 2000 | ||
| Log Wl = 0.53231logD2H – 0.96854 | 0.915 | Deng et al. 2000 | ||
| Other trees (D > 5.0) | W = 0.5834(D2H) – 8.151 | 0.9584 | Liu et al. 2009 | |
| Other trees (1.0 ≤ D ≤5.0) | W = 2.0141(D2H)0.889 | 0.9228 | Liu et al. 2009 | |
| Shrubs and tree seedlings | Rhamnus leptophylla Schneid | W = 0.9598(d2H)0.8849 | 0.9569 | Liu et al. 2009 |
| Lindera pulcherrima var. hemsleyana | W = 1.3295(d2H)0.835 | 0.9388 | Liu et al. 2009 | |
| Zanthoxylum ovalifolium var. spinifolium | W = 0.5379(d2H) + 29.405 | 0.9359 | Liu et al. 2009 | |
| Myrsine africana L. | W = 0.5757(d2H) + 18.309 | 0.8144 | Liu et al. 2009 | |
| Zanthoxylum esquirolii Levi., Zanthoxylum calcicola Huang | W = 1.1807(d2H) + 1.1754 | 0.9849 | Liu et al. 2009 | |
| Lindera communis | W = 2.2795(d2H)0.7636 | 0.9619 | Liu et al. 2009 | |
| Rhamnus heterophylla Oliv. | W = 0.9598(d2H)0.8849 | 0.9005 | Liu et al. 2009 | |
| Stachyurus obovatus (Rehd.) Hand.-Mazz. | W = 0.1867(d2H)1.2003 | 0.9406 | Liu et al. 2009 | |
| Others (D < 1.0) | W = 0.5418(d2H) + 17.287 | 0.7817 | Liu et al. 2009 | |
| 1.0 ≤ D ≤5.0 | W = 0.5834(D2H) – 8.151 | 0.9228 | Liu et al. 2009 | |
| D > 5.0 | W = 2.0141(D2H)0.889 | 0.9228 | Liu et al. 2009 | |
| Lianas and vines | D > 1.0 | Ws = 0.8745(D2H)0.7846 | 0.9278 | Yang et al. 2003 |
| Wl = 0.2556(D2H)0.8224 | 0.932 | Yang et al. 2003 | ||
| D ≤ 1.0 | Ln(W) = –1.423 + 2.155Ln(d) | 0.7364 | Yuan et al. 2009 | |
| W: Aboveground biomass; Wl: Leaf biomass; Ww: Wood biomass; Wt: Total biomass; Wr: Root biomass; Ws: Stem biomass; Wb: Branch biomass; D: Diameter at breast height; d: Basal diameter; H: Height or length | ||||
2.2.2 Belowground biomass
A quadrat (20 m × 20 m) close to and with habitat similar to the permanent plot was selected. A total of 25 soil pits, with an area of 50 cm × 50 cm each and 5 m interval in parallel, were dug into the bed rock (ca. 50–60 cm in depth). Soils were sampled at each 10 cm layer by using plastic bags and then washed in the laboratory to remove rocks. Roots in each soil layer were collected and separated into three root diameter classes: coarse root (root diameter ≥ 10 mm), medium root (2–10 mm), and fine root (≤ 2 mm). Fresh roots were weighed and dried in an oven for 48 h at a constant temperature of 85 °C and then weighed again.
2.2.3 Biomass of bryophytes and lichens
The bryophyte biomass was obtained from a karst forest in eastern central Guizhou province (Wang and Zhang 2010). Five quadrats (1 m × 1 m each) and 10 sub-quadrats (10 cm × 10 cm each) were randomly set up to collect bryophytes growing on rocks, soils, and woods. The total bryophyte biomass was 0.078 Mg ha−1.
Similarly, the lichen biomass was obtained from a 50-year old secondary subtropical forest in central Yunnan province (Li 2008). Five collection areas (4 m × 4 m each) of lichen litter were established in the forest, and lichen litter was collected once a month. Lichen biomass (0.043 Mg ha−1) was estimated by multiplying lichen litter in October by 100.
2.2.4 Necromass of woody debris and litter
Species, height (or length), relative position, and degree of decomposition of all woody debris, as well as DBH for dead standing trees and diameters at two ends for fallen dead woods in the plot, were recorded during the summer of 2013. The necromass of dead standing trees was predicted using their corresponding biomass models (Table 1). The necromass of fallen dead woods was calculated as the product of wood density (0.43–0.46 g cm−3) measured using 60 randomly selected fallen woods, and their volumes (Vl in m3) were calculated using the following equation:
where Dl was the diameter of large end of fallen woods, Ds was the diameter of small end of fallen woods, and l was the length of fallen woods.
Litter at the Tianlongshan site was inventoried by Liu et al. (2011). Three litter quadrats (40 cm × 40 cm each) were randomly selected in each microhabitat of the karst forest stand, namely, stone groove with richer soil, stone table with limited soil, and soil-rich slope. Litters were sampled at undecomposed, intermediately decomposed, and completely decomposed layers and then weighed after drying. The total litter necromass was estimated to be 8.6 Mg ha−1, which was calculated based on the weighted area of each microhabitat. This value was further used in the new estimate of this paper.
All biomass and necromass estimates were determined by considering the plot area projection. That is, the 2 ha plot had a non-horizontal projection area of 23333 m2. Statistical analyses were performed using Pearson correlation analysis (SPSS 19.0).
3 Results
The AGB of all trees in the 2 ha plot was 134.6 Mg ha−1. Five dominant tree species, namely, Lithocarpus confinis, Platycarya strobilacea, Itea yunnanensis, Machilus cavaleriei, and Carpinus pubescens, accounted for 92.4% (124.4 Mg ha−1) of the AGB of all trees. The AGB of shrubs and of lianas and vines were 1.8 and 0.9 Mg ha−1, respectively. Dominant shrubs and lianas and vines (DBH ≥ 1 cm) accounted for 1/3 of the total AGB of shrubs and lianas and vines, although the number of the corresponding individuals was only 1.6% of the total number of individuals. Other AGB components, including herbs (0.4 Mg ha−1), bryophytes (0.078 Mg ha−1), and lichens (0.043 Mg ha−1), were relatively low.
Each individual tree exhibited different AGBs ranging between 0.09 and 1213 kg per individual, and the average AGB of all tree individuals was 18.1 kg. All tree individuals were evenly distributed in the 2 ha plot, resulting in the even spatial distribution of AGB (Fig. 2a). However, different tree species showed different spatial distributions of their ABG. Lithocarpus confinis and Platycarya strobilacea contained the largest numbers of individuals and the highest AGBs of tree individual (25 and 25.8 kg per individual in average, respectively); thus, these species had the highest AGB in the plot. However, the AGB of Lithocarpus confinis was more (74.0%) distributed at the top half of the plot (Fig. 2b), whereas that of Platycarya strobilacea was highly (79.9%) distributed at the bottom half of the plot (Fig. 2c). Itea yunnanensis was less spatially distributed at the top-right corner; hence, the AGB was higher (61.2%) at the bottom and lower (38.8%) layer of the top half (Fig. 2d). The relatively smaller Machilus cavaleriei (7.8 kg AGB per individual in average) was mostly distributed at the top-left half of the plot and had 60.8% of their total AGB (Fig. 2e). The AGB of Carpinus pubescens was mainly gathered at the very top-right corner of the plot, where 94.1% of the total AGB was distributed (Fig. 2f).
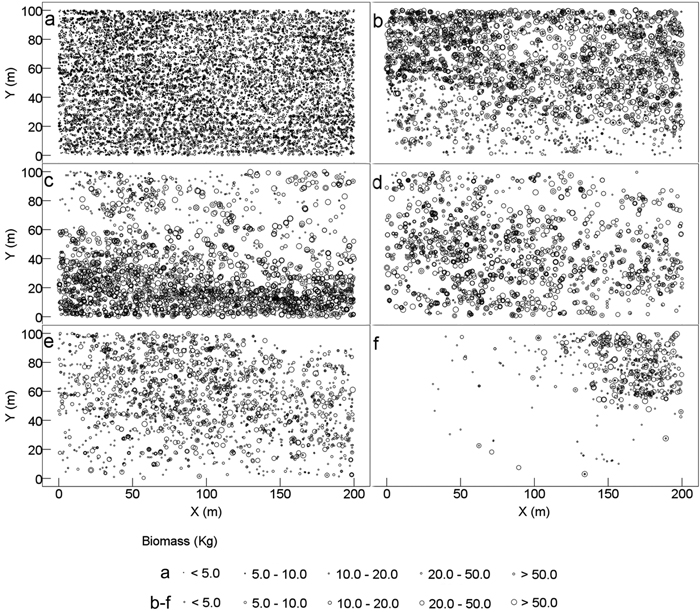
Fig. 2. Spatial distribution pattern of tree aboveground biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China. (a) all trees, (b) Lithocarpus confinis, (c) Platycarya strobilacea, (d) Itea yunnanensis, (e) Machilus cavaleriei, and (f) Carpinus pubescens. X: the distance from east to west of the plot; Y: the distance from south to north of the plot.
Shrubs (DBH ≥ 1 cm) had relatively small AGB (0.079–42.2 kg per individual with an average of 0.81 kg). Shrubs were more distributed at the middle to upper layer of the plot, where higher proportion of shrub biomass was found (Fig. 3a). Lianas and vines (DBH ≥ 1 cm) had even small AGB (0.067–28.7 kg per individual with an average of 2.1 kg). Approximately 74.9% of lianas and vines and 90% of their AGB were concentrated at the bottom half of the plot, especially for individuals with high biomass at the bottom-left and bottom-right corners (Fig. 3b).
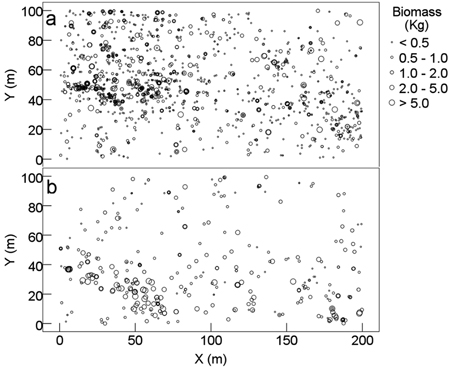
Fig. 3. Spatial distribution pattern of aboveground biomass of shrubs and lianas and vines of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China. (a) shrubs (DBH ≥ 1 cm) and (b) lianas and vines (DBH ≥ 1 cm). X: the distance from east to west of the plot; Y: the distance from south to north of the plot.
The BGB of the secondary forest growing in the plateau-surface karst terrain was estimated for the first time. The total BGB of the plot was 20.3 Mg ha−1. Coarse roots had higher biomass (11.0 Mg ha−1) than medium roots (5.9 Mg ha−1) and fine roots (3.3 Mg ha−1). The biomass of coarse, medium and fine roots all decreased with increasing soil depth (Fig. 4). Approximately 96.4% of the total BGB was distributed in the soil depth of less than 50 cm. More than 70% of total BGB (14.7 Mg ha−1) was allocated to the surface soil of 0–20 cm. This characteristic is true for roots in different diameter classes. The biomass of coarse roots with increasing depth (0–40 cm) was higher than that of medium and fine roots but became the lowest value when the soil depth was below 40 cm (Fig. 4).
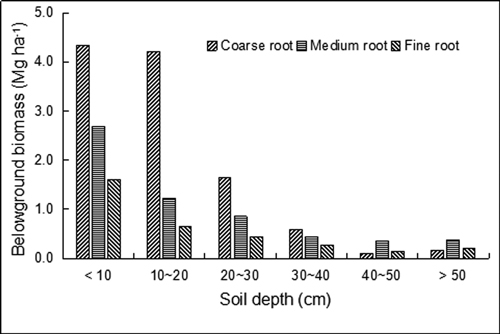
Fig. 4. Belowground biomass of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China. Coarse root (root diameter ≥ 10 mm), medium root (2–10 mm), and fine root (≤ 2 mm).
The BGB of coarse root in each soil pit ranged from 0to 19.0 kg m−3 (average = 2.6 ± 3.9 kg m−3), whereas those of medium and fine root ranged from 0.3 to 3.1 kg m−3 (average = 1.2 ± 0.7 kg m−3) and from 0.3 to 1.3 kg m−3 (average = 0.6 ± 0.3 kg m−3), respectively. The BGB in each soil pit significantly differed, with approximately 25-fold for the highest soil pit BGB (23.1 kg m−3) compared with the lowest (0.9 kg m−3), which was mainly caused by the random distribution of coarse roots. The BGB of each soil pit in the local habitats was not related to the spatial distribution of aboveground woody plants (Fig. 5) and soil pit depth (p > 0.05).
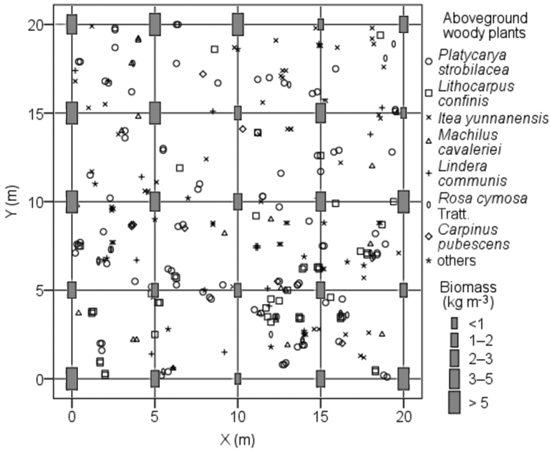
Fig. 5. Spatial distribution of aboveground woody plants and soil pits with its belowground biomass in root sampling quadrat of karst evergreen and deciduous broad-leaved mixed forest in central Guizhou province, southwestern China. X: the distance from west to east of the quadrat; Y: the distance from south to north of the quadrat.
All woody debris in the plot had 9.0 Mg ha−1 necromass, and the litter had 8.6 Mg ha−1. The aboveground green vascular plants (137.7 Mg ha−1) and roots (20.3 Mg ha−1) contributed mainly to the forest biomass. Estimated biomass of bryophytes (0.078 Mg ha−1) and lichens (0.043 Mg ha−1) was very low. In total, the evergreen and deciduous broad-leaved mixed forest in the plateau-surface-type karst terrain of central Guizhou province had 158.1 Mg ha−1 biomass and 17.6 Mg ha−1 necromass.
4 Discussion and conclusions
The AGB of trees and shrubs in this study (136.4 Mg ha−1) was significantly higher than the AGB (65.4–115.2 Mg ha−1) estimated by Liu et al. (2009) for the similar site by using the same biomass models (Table 2). This variation was mainly attributed to differences in plot area calculation. The plot area in this study was calculated by horizontal projection according to the average slope of the plot. The 2 ha plot was equal to 23 333 m2 in non-horizontal projection. However, Liu et al. (2009) did not consider such projection (horizontal projection) and still used the small plot area (600 m2) of the original inclined hill slope. Conversely, the AGB of trees and shrubs in this study should be 116.9 Mg ha−1 if we did not consider area projection, which was close to the estimate by Liu et al. (2009). However, the large plot exhibited advantage for accurate estimation of forest biomass.
| Table 2. Comparison of biomass between karst and nonkarst forests in southern China. View in new window/tab. |
Forest biomass in different karst morphological types varied to a certain extent. The biomass of green vascular plants of the secondary forest in plateau-surface-type karst terrain in central Guizhou province estimated in this study was similar to that of trough-valley-type karst forest in northern Guizhou (Zhong et al. 2014) and slightly higher than that of peak-forest plain-type karst forest in northwestern Guangxi (Zeng et al. 2007). However, the AGB and BGB of the secondary forest in the plateau-surface-type karst were lower than those of primary forests in the peak-clump depression-type karst in southern Guizhou (Zhu et al. 1995; Luo et al. 2010; Ni et al. 2015). This finding was due to the fact that the former forest was cut off ca. 55 years ago and is currently under the succession stage of subclimax. By contrast, the latter one was in the climax stage with higher DBH and tall trees (Zhu et al. 1995; Ni et al. 2015), as well as significantly different species composition (Zhang et al. 2012). Karst forest had lower AGB and BGB (Table 2) than the typical evergreen broad-leaved forests in nonkarst region in the same climate zone (Qiu et al. 1984; Chen et al. 1993; Huang 2006; Yang et al. 2010).
Biomass models have been widely used to estimate forest biomass (Lieth and Whittaker 1975; Brown and Lugo 1982), but karst forests had limited numbers of biomass models, the majority of which focused on forest plantations (Zhang and Yuan 1988; Ding et al. 2003). A unified biomass model was established for natural forests and secondary mixed forests to estimate the biomass of all woody species (Yang and Cheng 1991; Zhu et al. 1955; Yu et al. 2002); and few biomass models were established for several dominant species (Liu et al. 2009; Liu et al. 2013). Previous biomass estimates may present bias with overestimates or underestimates. In this study, we used as many biomass models as possible, for example, the locally available models for major tree and shrub species obtained from previous studies at the same site (Liu et al. 2009; Liu et al. 2013), models for species from the same genus or family, models from the same species outside the karst terrain, and unified models from karst or nonkarst terrain but in the same climate zone (Table 1). Using biomass models from other forest stands outside the large plot could be an alternative choice because the study plot and its surrounding area have been so far protected by the local government. Thus, further cutting of sample trees and more biomass model establishment for more species have been impossible at this forest stand. This limitation might influence the accuracy of biomass predictions for some unimportant species but did not affect the major species and the entire plot.
The necromass of dead standing trees was probably overestimated since wood decay caused loss of necromass before snags (standing dead trees) fell down and the necromass of litter and biomass of bryophytes and lichen were obtained from studies conducted inside or outside the karst forest stand. However, these components had minimal necromass or biomass. Thus, such treatment could not considerably affect the estimate of biomass of the entire plot, although sub-sampling method should be conducted in future studies.
The distribution of woody species in karst forests, which was strongly related to the habitat heterogeneity, was spatially aggregated (Zhang et al. 2013). However, the spatial distribution of biomass is not simply determined by the spatial distribution of species. This characteristic could be accurately determined by the spatial distribution of individuals of dominant trees with superior DBH, tall height, and dense wood. This parameter is also strongly related to the high heterogeneity of karst terrain. Rocky microhabitats and shallow soils constrain the tree growth and even further reduce the biomass of tree individuals in some places where tree species are aggregately distributed.
The unique habitats in karst region cause distinct biomasses and distribution patterns of roots between karst and nonkarst forests. Nevertheless, the rocky habitats also lead to the difficulty in obtaining root samples and further limit studies on belowground processes and biomass estimation of karst forests. The soil pit method used in this study is simple and relatively easy to perform; hence, this method could be used to effectively study the BGB of karst forests. However, the BGB variation among soil pits for coarse, medium and fine roots indicated the soil pit method was more accurate for inventorying fine root biomass than that of medium and coarse roots. The BGB and soil depth relationship (Fig. 4) indicated that the spatial pattern of root distribution was extremely complex because of the high heterogeneity of rock–soil landscape in the karst terrain. This characteristic considerably hindered the separation of roots among species and the harvest of entire roots (Ni et al. 2015). The total BGB could only be obtained and the BGB was likely underestimated in this study. The sampling area and number of soil pits should be considerably increased in future studies to represent the complex habitat heterogeneity and enhance the accuracy of BGB estimate. The soil pit method is further recommended to be used in combination with other methods, such as root coring and allometric equations (Park et al. 2007).
Forests worldwide sequester atmospheric carbon to biomass through photosynthesis and play a vital role in mitigating global climate change (Melillo et al. 1993; McGuire et al. 1993; Woodward et al. 1995; Schimel et al. 2001). The karst forest ecosystem in southwestern China is extremely fragile to human disturbances, and rocky desertification often occurred when forests were destroyed (Jiang et al. 2014). Potential restoration requires a long time, and in most cases, only grass, tussock, and shrub communities, rather than forests, are recovered. Degraded vegetation occupies 52.5% of the total vegetation area in Guizhou (ECVMC 2007). The biomass carbon in karst terrain of southwestern China could increase substantially if all these vegetations with low biomasses are converted into secondary forests or into climax forests, because biomass increased from degraded vegetation types such as grasslands, tussocks and shrublands to forests (Liu et al. 2013; Ni et al. 2015). The remarkable carbon storage potential in this region may contribute to the maintenance of regional carbon balance and mitigation of climate change. Site-based biomass estimates in karst vegetation are therefore not only important for providing data to benchmark global and regional vegetation and carbon models (Luyssaert et al. 2007; Kelley et al. 2013) but also for regional carbon inventory and vegetation restoration. Planting many trees and recovering numerous karst forests could potentially combat rocky desertification, enrich the local people, and sustain the regional environment in southwestern China.
Acknowledgements
This work was supported by the National Basic Research Program of China (2013CB956704), the Hundred Talents Program of the Chinese Academy of Sciences (2011031), and the Science and Technique Foundation of Guizhou Province (GKH-J-2012-2332). We thank the Puding Karst Ecosystem Research Station for assistance in all field works.
References
Alexeyev V., Birdsey R., Stakanov V., Korotkov T. (1995). Carbon in vegetation of Russian forests: methods to estimate storage and geographical distribution. Water Air Soil Pollution 82(1): 271–282. http://dx.doi.org/10.1007/BF01182840.
Browm S., Lugo A.E. (1982). The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 14: 161–187. http://dx.doi.org/10.2307/2388024.
Chen Q.H. (1982–2004). Flora Guizhouensis. Guizhou Science and Technology Publishing House, Guiyang. [In Chinese].
Chen Z.H., Zhang H.D., Wang B.S., Zhang Z.Q. (1993). Studies on biomass and its allocation of the evergreen broadleaved forest in Heishiding, Guangdong. Acta Phytoecologica et Geobotanica Sinica 17: 289–298. [In Chinese]. http://dx.doi.org/10.3321/j.issn:1005-264X.1993.04.011.
Condit R. (1995). Research in large, long-term tropical forest plot. Trends in Ecology & Ecolution 10: 18–22. http://dx.doi.org/10.1016/s0169-5347(00)88955-7.
Deng S.J., Liao L.P., Wang S.L., Gao H., Lin B. (2000). Bioproductivity of Castanopsisi hysrix–Cyclobalanopsis glauca–Machilus pauhoi community in Huitong, Hunan. Chinese Journal of Applied Ecology 11: 651–654. [In Chinese]. http://dx.doi.org/10.13287/j.1001-9332.2000.0159.
Ding G.J. (2003). Study on biomass and productivity of Masson Pine planting stand. I. Biomass and density effect of different planting density. Journal of Fujian College of Forestry 23: 34–38. [in Chinese]. http://dx.doi.org/10.13324/j.cnki.jfcf.2003.01.009.
Dixon R.K., Brown S., Houghton R.A., Solomon A.M., Trexler M.C., Wisniewski J. (1994). Carbon pools and flux of global forest ecosystem. Science 263: 185–190. http://dx.doi.org/10.1126/science.263.5144.185.
Editorial Committee of Vegetation Map of the People’s Republic of China (ECVMC), Chinese Academy of Sciences. (2007). Vegetation map of the People’s Republic of China (1:1 000 000). Geology Press, Xi’an. [In Chinese].
Feng Z.W., Wang X.K., Wu G. (1999). Biomass and primary productivity of forest ecosystems in China. Science Press, Beijing. 241 p. [In Chinese].
He H.Z., Huang L.H., Duan X., He R.K. (2007). Study on biomass in main afforestation tree species of the second ring forest-belt of Guiyang. Guizhou Science 25: 33–39. [In Chinese]. http://dx.doi.org/10.3969/j.issn.1003-6563.2007.03.006.
Huang D.Z. (2006). Biomass characteristics of secondary forest community of Cyclobalanopsis chungii in the lower Minjiang River. Protection Forest Science and Technology 70: 16–18. [In Chinese]. http://dx.doi.org/10.13601/j.issn.1005-5215.2006.01.007.
Jiang Z.C., Lian Y.Q., Qin X.Q. (2014). Rocky desertification in Southwest China: impacts, causes, and restoration. Earth–Science Reviews 132: 1–12. http://dx.doi.org/10.1016/j.earscirev.2014.01.005.
Kelley D.I., Prentice I.C., Harrison S.P., Wang H., Simard M., Fisher J.B., Willis K.O. (2013). A comprehensive benchmarking system for evaluating global vegetation models. Biogeosciences 10: 3313–3340. http://dx.doi.org/10.5194/bg-10-3313-2013.
Li S. (2008). Species diversity, biomass and distribution of epiphytic lichens in primary and secondary forests in Ailao Mountains, Yunnan. Master Thesis, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. [In Chinese].
Lieth H., Whittaker R.H. (1975). Primary productivity of the biosphere. New York, Springer. http://dx.doi.org/10.1007/978-3-642-80913-2.
Liu C.C., Wei Y.F., Liu Y.G., Guo K. (2009). Biomass of canopy and shrub layers of karst forest in Puding, Guizhou, China. Chinese Journal of Plant Ecology 33: 698–705. [In Chinese]. http://dx.doi.org/10.3773/j.issn.1005-264x.2009.04.008.
Liu Y.G., Liu C.C., Li G.Q., Wei Y.F., Liu Y.G., Guo K. (2011). Litter mass of five karst forests and their hydrological effects in Guizhou. Scientia Silvae Sinicae 47: 82–88. [in Chinese]. http://dx.doi.org/10.11707/j.1001-7488.20110313.
Liu Y.G., Liu C.C., Wang S.J., Guo K., Yang J., Zhang X.S., Li G.Q. (2013). Organic carbon storage in four ecosystem types in the karst region of southwestern China. PLoS ONE 8: e56443. http://dx.doi.org/10.1371/journal.pone.0056443.
Luo D.H., Xia J., Yuan J.W., Zhang Z.H., Zhu J.D., Ni J. (2010). Root biomass of karst vegetation in a mountainous area of southwestern China. Chinese Journal of Plant Ecology 34: 611–618. [In Chinese]. http://dx.doi.org/10.3773/j.issn.1005-264x.2010.05.015.
Luyssaert S., Inglima I., Jung M., Richardson A.D., Reichstein M., Papale D., Piao S.L., Schulze E.-D., Wingate L., Matteucci G., Aragao L., Aubinet M., Beer C., Bernhofer C., Black K.G., Bonal D., Bonnefond J.-M., Chambers J., Ciais P., Cook B., Davis K.J., Dolman A.J., Gielen B., Goulden M., Grace J., Granier A., Grelle A., Griffis T., Grünwald T., Guidolotti G., Hanson P.J., Harding R., Hollinger D.Y., Hutyra L.R., Kolari P., Kruijt B., Kutsch W., Lagergren F., Laurila T., Law B.E., LeMaire G., Lindroth A., Loustau D., Malhi Y., Mateus J., Migliavacca M., Misson L., Montagnani L., Moncrieff J., Moors E., Munger J.W., Nikinmaa E., Ollinger S.V., Pita G., Rebmann C., Roupsard O., Saigusa N., Sanz M.J., Seufert G., Sierra C., Smith M.-L., Tang J., Valentini R., Vesala T., Janssens I.A. (2007). CO2 balance of boreal, temperate, and tropical forests derived from a global database. Global Change Biology 13: 2509–2537. http://dx.doi.org/10.1111/j.1365-2486.2007.01439.x.
McGuire A.D., Joyce L.A., Kicklighter D.W., Melillo J.M., Esser G., Vorosmarty C.J. (1993). Productivity responses of climax temperate forests to elevated temperature and carbon dioxide: a North American comparison between two global models. Climatic Change 24: 287–310. http://dx.doi.org/10.1007/BF01091852.
Melillo J.M., McGuire A.D., Kicklighter D.W., Moore III B., Vorosmarty C.J., Schloss A.L. (1993). Global climate change and terrestrial net primary production. Nature 363: 234–240. http://dx.doi.org/10.1038/363234a0.
Ni J. (2003). Net primary productivity in forests of China: scaling-up of national inventory data and comparison with model predictions. Forest Ecology and Management 176: 485–495. http://dx.doi.org/10.1016/S0378-1127(02)00312-2.
Ni J., Luo D.H., Xia J., Zhang Z.H., Hu G. (2015). Vegetation in karst terrain of southwestern China allocates more biomass to roots. Solid Earth 6: 799–810. http://dx.doi.org/10.5194/se-6-799-2015.
Ni J., Zhang X.S., Scurlock J.M.O. (2001). Synthesis and analysis of biomass and net primary productivity in Chinese forests. Annals of Forest Science 58: 351–384. http://dx.doi.org/10.1051/forest:2001131.
Park B.B., Yanai R.D., Vadeboncoeur M.A., Hamburg S.P. (2007). Estimating root biomass in rocky soils using pits, cores, and allometric equations. Soil Science Society of America Journal 71: 206–213. http://dx.doi.org/10.2136/sssaj2005.0329.
Powell S.L., Cohen W.B., Healey S.P., Kennedy R.E., Moisen G.G., Pierce K.B., Ohmann J.L. (2010). Quantification of live aboveground forest biomass dynamics with Landsat time-series and field inventory data: a comparison of empirical modeling approaches. Remote Sensing of Environment 114: 1053–1068. http://dx.doi.org/10.1016/j.res.2009.12.018.
Qiu X.Z., Xie S.C., Jing G.F. (1984). A preliminary study on biomass of Lithocarpus xylocarpus forest in Xujiaba region, Ailao Mts., Yunnan. Acta Botanica Yunnanica 6(1): 85–92. [In Chinese].
Schimel D.S., House J.I., Hibbard K.A., Bousquet P., Ciais P., Peylin P., Braswell B.H., Apps M.J., Baker D., Bondeau A., Canadell J., Churkina G., Cramer W., Denning A.S., Field C.B., Friedlingstein P., Goodale C., Heimann M., Houghton R.A., Melillo J.M., Moore III B., Murdiyarso D., Noble I., Pacala S.W., Prentice I.C., Raupach M.R., Rayner P.J., Scholes R.J., Steffen W.L., Wirth C. (2001). Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 414: 169–172. http://dx.doi.org/10.1038/35102500.
Tu Y.L., Yang J. (1995). Study on biomass of the karst scrub community in central region of Guizhou Province. Carsologica Sinica 14: 199–208. [In Chinese].
Wang Z.H., Zhang Z.H. (2010). A study on biomass of bryophyte communities at the karst forest ecosystem in Mt. Yuntai, Guizhou Province. Journal of Guizhou Normal University (Natural Sciences) 28: 88–91. [In Chinese]. http://dx.doi.org/10.3969/j.issn.1004-5570.2010.04.020.
Woodward F.I., Smith T.M., Emanuel W.R. (1995). A global land primary productivity and phytogeography model. Global Biogeochemical Cycles 9: 471–490. http://dx.doi.org/10.1029/95GB02432.
Yang H.K., Cheng S.Z. (1991). Study on biomass of the karst forest community in Maolan, Guizhou Province. Acta Ecologica Sinica 11: 307–312. [In Chinese]. http://dx.doi.org/10.3321/j.issn:1000-0933.1991.04.010.
Yang Q.P., Li M.G., Wang B.S., Li R.W., Wang C.W. (2003). Dynamics of biomass and net primary productivity in succession of south subtropical forests in southwest Guangdong. Chinese Journal of Applied Ecology 14: 2136–2140. [In Chinese]. http://dx.doi.org/10.13287/j.1001-9332.2003.0471.
Yang T.H., Song K., Da L.J., Li X.P., Wu J.P. (2010). The biomass and aboveground net primary productivity of Schima superba–Castanopsis carlesii forest in east China. Science China Life Science 53: 811–821. http://dx.doi.org/10.1007/s11427-010-4021-5.
Yuan C.M., Liu W.Y., Li X.S., Yang G.P. (2009). Aboveground biomass of lianas and its response to anthropogenic disturbances in moist evergreen broad-leaved forests in the Ailao Mountains of southwestern China. Chinese Journal of Applied Ecology 33: 852–859. [In Chinese]. http://dx.doi.org/10.3773/j.issn.1005-264x.2009.05.003.
Yu L.F., Zhu S.Q., Ye J.Z., Wei L.M., Chen Z.R. (2002). Dynamics of a degraded karst forest in the process of natural restoration. Scientia Silvae Sinicae 38: 1–7. [In Chinese]. http://dx.doi.org/10.11707/j.1001-7488.20020101.
Zeng F.P., Peng W.X., Song T.Q., Wang K.L., Wu H.Y., Song X.J., Zeng Z.X. (2007). Changes in vegetation after 22 years’ natural restoration in the karst disturbed area in northwest Guangxi. Acta Ecologica Sinica 27: 5110–5119. [In Chinese]. http://dx.doi.org/10.3321/j.issn:1000-0933.2007.12.020.
Zhang J.Z., Yuan Y.Z. (1988). A study on the biomass and production of Pinus fenzeliana forest in Hainan. Acta Phytoecologicaet Geobotanica Sinica 12: 63–69. [In Chinese].
Zhang Z.H., Hu G., Zhu J.D., Ni J. (2012). Stand structure, woody species richness and composition of subtropical karst forests in Maolan, south-west China. Journal of Tropical Forest Science 24: 498–506.
Zhang Z.H., Hu G., Zhu J.D., Ni J. (2013). Aggregated spatial distributions of species in a subtropical karst forest, southwestern China. Journal of Plant Ecology 6: 131–140. http://dx.doi.org/10.1093/jpe/rts027.
Zhong Y.X., Zhou Y.C., Li Z.J. (2014). Research on the carbon storage and potential carbon sequestration of vegetation in the trough valley of a karst area, Yinjiang. Earth and Environment 42: 82–89. [In Chinese]. http://dx.doi.org/10.14050/j.cnki.1672-9250.2014.01.018.
Zhou G.S., Wang Y.H., Jiang Y.L., Yang Z.Y. (2002). Estimating biomass and net primary production from forest inventory data: a case study of China’s Larix forests. Forest Ecology and Management 169: 149–157. http://dx.doi.org/10.1016/S0378-1127(02)00305-5.
Zhu S.Q., Wei L.M., Chen Z.R., Zhang C.G. (1995). A preliminary study on biomass components of karst forest in Maolan of Guizhou Province, China. Acta Phytoecologica Sinica 19: 358–367. [In Chinese]. http://dx.doi.org/10.3321/j.issn:1005-264X.1995.04.011.
Total of 45 references.

