Effect of arginine-phosphate addition on early survival and growth of Scots pine, Norway spruce and silver birch
Häggström B., Lutter R., Lundmark T., Sjödin F., Nordin A. (2023). Effect of arginine-phosphate addition on early survival and growth of Scots pine, Norway spruce and silver birch. Silva Fennica vol. 57 no. 2 article id 22013. https://doi.org/10.14214/sf.22013
Highlights
- Arginine-phosphate addition (APA) represents a potential tool to aid regeneration of planted trees, especially to increase survival of Scots pine seedlings on sites where susceptible to pests
- Effects of APA however varies between different sites.
Abstract
Applying arginine-phosphate (AP) to tree seedlings at planting is a novel silvicultural practice in Northern Europe to improve the success of forest regeneration. We present three case-studies of the potential advantages of adding AP at planting on the establishment and damage susceptibility of seedlings in pure and mixed plantings of Scots pine (Pinus sylvestris L.), Norway spruce (Picea abies (L.) H. Karst. ) and silver birch (Betula pendula Roth) over two years in the field. Location of study sites were in southern (S), northeastern (NE) and northwestern (NW) Sweden. The main agents of damage were pine weevil (Hylobius abietis L.) on conifers at the south site, browsing of birch at all sites and browsing/other top damage to conifers at the north sites. The effect of adding AP varied between the sites. It was positive for survival of pine at site S, despite considerable damage by pine weevil. However, at the S site more of the surviving spruce and birch were browsed when treated with AP. At the NE site AP-treatment had positive effects on conifer growth. At the NW site adding AP positively affected survival and growth of all three species, and AP-treated seedlings of all species were less browsed than untreated seedlings. AP treatment presents a potential tool to improve the success of forest regeneration, especially when establishing pine stands in south Sweden.
Keywords
Pinus sylvestris;
Betula pendula;
Picea abies;
forest regeneration;
seedling growth;
seedling survival;
arginine
-
Häggström,
Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences, 90183 Umeå, Sweden
 https://orcid.org/0000-0002-7738-5493
E-mail
bodil.haggstrom@slu.se
https://orcid.org/0000-0002-7738-5493
E-mail
bodil.haggstrom@slu.se

-
Lutter,
Institute of Forestry and Engineering, Estonian University of Life Sciences, Kreutzwaldi 5, Tartu 51006, Estonia
 https://orcid.org/0000-0001-5847-4282
E-mail
reimo.lutter@emu.ee
https://orcid.org/0000-0001-5847-4282
E-mail
reimo.lutter@emu.ee
-
Lundmark,
Department of Forest Ecology and Management, Swedish University of Agricultural Sciences, 90183 Umeå, Sweden
 https://orcid.org/0000-0003-2271-3469
E-mail
tomas.lundmark@slu.se
https://orcid.org/0000-0003-2271-3469
E-mail
tomas.lundmark@slu.se
- Sjödin, Unit for field-based forest research, Swedish University of Agricultural Sciences, 90183 Umeå, Sweden E-mail fredrik.sjodin@slu.se
-
Nordin,
Umeå Plant Science Centre, Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences, 90183 Umeå, Sweden
 https://orcid.org/0000-0002-5765-3550
E-mail
annika.nordin@slu.se
https://orcid.org/0000-0002-5765-3550
E-mail
annika.nordin@slu.se
Received 18 October 2022 Accepted 9 August 2023 Published 5 September 2023
Views 71900
Available at https://doi.org/10.14214/sf.22013 | Download PDF

Supplementary Files
1 Introduction
In the Nordic countries, forest regeneration practice mainly involves planting of nursery grown Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) H. Karst.) seedlings as single species stands. However, the two most common broadleaved tree species, downy birch (Betula pubescens Ehrh.) and silver birch (B. pendula Roth) are increasingly becoming more important commercially. The distribution between the two birch species varies in different regions of Sweden (Dahlgren Lidman 2022) and are generally not separated, but commonly referred to as “birch” in Swedish forest statistics (Skogsdata 2021). Birch is in general naturally regenerated in Sweden (Skogsdata 2021), but when planted it is commonly nursery grown silver birch since this birch species grow faster and have a higher timber quality than downy birch (Heräjärvi 2001; Rytter et al 2014).
The success of forest regeneration in the shortest possible time is important both to reach aimed production goals and to shift the harvested area from a carbon source to a carbon sink. Newly planted seedlings have limited access to water and nutrients before their roots extend into the soil of the planting site and thereby face challenges of drought and slow initial growth. They are also highly susceptible to damage by biological agents in this stage both due to their small size and because their chemical defense is limited until they have reached a balance between resource availability and needs. Damage from pine weevil (Hylobius abietis L.) is the most common biological agent of damage for newly planted tree seedlings in the Nordic countries. Pine weevils are attracted to newly harvested conifer forest where they reproduce and feed on the bark of twigs and seedlings of pine and spruce, while they are less attracted to broadleaved species (Day et al. 2004; Löf et al. 2004; Månsson and Schlyter 2004). The loss of bark hampers seedling growth, and if the damage becomes severe, the seedling will die (Örlander 1990; Thorsen et al. 2001). Once the small seedlings have outgrown the risk of severe damage by pine weevil, browsing by cervids is the next threshold to overcome. The most common browsing cervids in Sweden are roe deer (Caprerolus capreoulus L.) and moose (Alces alces L.) that both frequently damage young trees (Bergqvist 2017). The level of damage cause by each cervid species on each tree species depends on many factors, such as the local cervid population density, the local quantity and quality of the tree species as well as availability of other food sources and shelter (Gill 1992; Niemelä and Danell 1988; Bergqvist et al. 2014; Pfeffer et al. 2022). Moose is often in focus when browsing damage is studied (Wallgren et al. 2013; Bergqvist et al. 2014; Pfeffer et al. 2022) and the national browsing damage inventory ÄBIN stands for “älgbetesinventering” (= moose-browsing inventory). Due to the larger size, a moose can reach higher and consume more than a roe deer. Moose can also easier cope with greater snow depths is therefore the dominant species in the northern Sweden. The winter diet of moose is dominated by pine (Felton et al. 2020), which is the dominant conifer species in north Sweden. However, in south Sweden row deer populations are larger along with increasing populations of red deer (Cervus elaphus L.) and locally fallow deer (Dama dama L.), the two latter can also utilize various tree species in their diet (Roberge 2012; Bergqvist 2017). Broadleaved species are generally preferred by all browsing cervids, although pine is also frequently browsed during winter by both moose and roe deer (Bergqvist 2017). Silver birch is preferred over downy birch (Danell et al. 1985) and in the north part of Sweden the natural composition is dominated by downy birch (Dahlgren Lidman 2022), which make planted silver birch more attractive as forage than the natural regenerated birch in this area. Spruce is generally less browsed but can also suffer from such damages (Vehviläinen and Koricheva 2006).
One option to enhance early growth and survival of seedlings is to add a slow-release nitrogen source at planting (Brand 1991; Thiffault and Jobidon 2006). Forest growth is often limited by low nitrogen availability in boreal regions (Tamm 1991; Grossnickle 2000; Bhatti et al. 2006) where nitrogen is mainly available to seedlings in its natural organic form i.e., amino acids (Inselsbacher et al. 2011). Conifers synthesize the amino acid arginine as an internal nitrogen store (Nordin et al. 2001); this and other amino acids can be taken up by trees through their roots (Öhlund and Nasholm 2001; Gruffman et al. 2013). A commercially available nitrogen source – arGrow® Granulat (Arevo AB, Umeå, Sweden) has been developed in which arginine is crystallized with phosphate and formulated as slow-release granules. As arginine is a strong cation, it binds to soil particles (Inselsbacher et al. 2011) and is thus retained in the soil and prevented from leaching away (Hedwall et al. 2018). Pine and spruce seedlings grown on arginine in nurseries have shown greater development of fine roots as well as increased mycorrhizal colonization of these roots compared to seedlings grown on inorganic fertilizers (Gruffman et al. 2012). The early root growth is essential for successful establishment of a newly planted seedling (Burdett 1990; Grossnickle 2005); moreover, mycorrhiza further increase the ability of seedlings to take up water and nutrients (Bréda et al. 2006; Brunner et al. 2015). ArGrow®, i.e., arginine phosphate (AP), has in recent years been commercially used with the intention that the extra cost at planting will be financially returned in the form of increased early survival and improved growth so that more of the planted trees will be a part of the future stand. The planted material in Sweden is generally grown from a seed mix from open pollinated seed orchards, i.e., with at least one parent being one chosen to give improved performance compared to the average naturally generated trees. With the faster initial growth and the genetic benefit gained from higher survival of the planted seedlings as compared to the naturally regenerated trees that become incorporated and kept in the stand where planted seedlings perish, the rotation period is expected to shorten as well. So far, there are only few studies in field conditions to test the effect of AP on forest generation success, where it has been demonstrated that survival and early growth after planting and sowing of Scots pine seedlings in north Sweden can be enhanced by the addition of AP (Castro et al. 2021; Häggström et al. 2021; Domevščik et al. 2023). However, it is not known if the same effect might occur in this species in southern Sweden; nor if the regeneration of Norway spruce and silver birch might be equally enhanced. The aim of this study is to provide further knowledge for forest owners as to how the use of AP could be beneficial not only for Scots pine, but also for Norway spruce and silver birch.
In our present three-site case-study, we investigate the effect of AP treatment on early performance of Scots pine, Norway spruce and silver birch and the potential for an AP treatment to affect their susceptibility to their most common biological agents of damage, i.e., pine weevil on conifers, and ungulates browsing on birch. For this experiment we utilized a setting that was intended for long-term monitoring of different species compositions, where one site in the south and two sites in the north of Sweden were regenerated with Scots pine, Norway spruce and silver birch seedlings, with and without the addition of AP. This first study does not consider the effects of competition for resources in the different species compositions, since the trees are yet too small to reveal these competitive interactions of tree species mixing. However, the data from this study set a baseline for the future study by providing the original variability in performance due to differing site conditions. Nevertheless, we use this opportunity to quantify the levels of damage caused by pine-weevil in different species compositions in comparison with the previously known preference patterns of this common agent of damage. Our hypotheses were that: 1) Adding AP enhances the survival and growth of all three tree species similarly at all three sites; and 2) Adding AP increases the vitality of seedlings enabling them to withstand damage from their local pests, better than seedlings with no AP added.
2 Material and methods
2.1 Experimental sites and setup
The tree species included in the trial are Scots pine, Norway spruce and silver birch. Each of the three sites included in this study comprised 14 plots. Seedlings in seven of the plots were treated with a single dose of arginine-phosphate formulated as arGrow® Granulat (Arevo AB, Umeå, Sweden). Each dose contained 40 mg N and 22 mg P, the active substance being L-arginine phosphate (C6H17N4O6P). The species composition differed among each of the seven treated and untreated plots as follows: 1) only pine; 2) only spruce; 3) only birch; 4) pine + spruce; 5) pine + birch; 6) spruce + birch; and 7) pine + spruce + birch. Thus, each species occurred in four untreated and four AP-treated plots and formed our experimental replicates. In the mixed plots, the different species were planted alternately. All plots were 24 m × 24 m surrounded by a buffer zone of the same species as in the plot, making a total area of 35 m × 35 m.
All seedlings were grown in container systems in nurseries and freezer stored. All seedlings were handled according to common practice regarding transport and thawing of freezer stored seedlings, irrigation prior to planting was made when needed. All seedlings were vital at planting. For each site, all seedlings of the same species were of the same type and of the most appropriate commercially available proveniences (Table 1.) Planting was carefully made by experienced planters with Pottiputki planting pipes according to recommended practice, i.e., deep enough for the substrate to be covered by soil when compacted around by pushing firmly but carefully with one foot after planting. For the AP-treatment, a Pottiputki with an attached arGrow® dispenser (commercially designed for this purpose and used in practice) was used. One dose is dropped into the pipe when it is inserted in the ground and prior to putting the plant in the pipe, for the dose to end up directly below the seedlings’ roots. The south site was planted 17th of June 2020, the northeast site 22nd of June 2020 and the northwest site 17th of June 2019 (Table 1).
| Table 1. Description of the conditions at the three study sites located in the south, northeastern and northwestern parts of Sweden respectively, including plot setup, number of seedlings planted and seedling material details for the three sites and for Scots pine, Norway spruce and silver birch respectively. Growing season, mean annual temperature and mean annual precipitation extrapolated for the Swedish Meteorological and Hydrological Institute (SMHI) maps showing normal length of vegetation period 1981–2010 (SMHI 2022a-d). All seedlings were containerized. *Theoretical numbers since the true number for each row sometimes deviated depending on availability of planting positions. **Approximate mean sizes common for the container system, not actual measurements of the seedlings. | ||||
| Site | South (S) site | Northeast (NE) site | Northwest (NW) site | |
| Coordinates | 57.193157,14.821763 | 64.162174, 19.661918 | 64.102337, 18.149540 | |
| Elevation (m. a.s.l) | 225–230 | 160–165 | 295–300 | |
| Trial establishment date | June 17th 2020 | June 22nd 2020 | June 17th 2019 | |
| Landowner | Sveaskog | Private | Sveaskog | |
| Growing season (days) | 190–200 | 150–160 | 140–150 | |
| Coldest month avg. T (°C) | –1 to –2 (January) | –7 to –8 (January | –9 to –10 (January) | |
| Warmest month avg. T (°C) | +16 to +17 (July) | +15 to +16 (July) | +14 to +15 (July) | |
| Mean annual T (°C) | 6–7 | 2–3 | 1–2 | |
| Mean annual precipitation (mm) | 600–800 | 600–800 | 400–600 | |
| Moist class | mesic to dry | mesic | dry–mesic | |
| Harvested stand | spruce | spruce | pine | |
| Landscape | Hummocky, but with relatively flat plot areas | From flat to moderate W slope | Ridges, from flat to gentle slope | |
| Vegetation type | Grass + Rubus idaeus L. | Vaccinium myrtillus L. | Vaccinium myrtillus L. + Vaccinium vitis-idaea L. | |
| Vegetation 2nd season | Very rich | Moderate | Sparse | |
| Tot. size harvested area (ha) | 21 | 5 | 20 | |
| Seedlings per plot* | 9 × 9 | 9 × 9 | 12 × 12 | |
| Seedling spacing (m) | 2.6 × 2.6 | 2.6 × 2.6 | 2 × 2 | |
| Seedling material | ||||
| Pine | Age (yr) | 1 | 1 | 2 |
| Nursery | Lilla Istad, SSP | Skogforsk, Sävar | Skadom nursery | |
| Seed origin | Seed orchard | Seed orchard | Seed orchard | |
| Provenience | 30-G7 Lilla Istad | FP-T8 Dal | FP 625 T8 Dal | |
| Container system | S50 | Hiko VAB90 | SP90 | |
| Height (cm)** | 13 | 13.2 | 25 | |
| Stem base (mm)** | 2.45 | 3.1 | 5 | |
| Pine-weevil protection | yes, conniflex | no | no | |
| Spruce | Age (yr) | 1.5 | 1 | 2 |
| Nursery | Trekantens nursery, SSP | Skogforsk, Sävar | Skadom nursery | |
| Seed origin | Seed orchard | Seed orchard | Forest | |
| Provenience | FT-907 Ekebo | FP-130 Domsjöänget | Fullsborn 61*25 | |
| Container system | Svepot 115 | Hiko VAB90 | SP90 | |
| Height (cm)** | 30.5 | 24.4 | 30 | |
| Stem base (mm)** | 4.6 | 3.1 | 6 | |
| Pine-weevil protection | yes, conniflex | no | no | |
| Birch | Age (yr) | 1 | 1 | 1 |
| Nursery | Mellanå Plant AB | Skogforsk, Sävar | Skadom nursery | |
| Seed origin | Seed orchard | Seed orchard | Seed orchard | |
| Provenience | Ekebo 5 B | Lat 62–64° (Finland) | SV 413 Oitti (Finland) | |
| Container system | PLEK 36 | Plantek 49F | SP120 | |
| Height (cm)** | 40–80 | N.A. | 40 | |
| Stem base (mm)** | N.A. | N.A. | 6 | |
The three sites represent commonly occurring soil conditions in Swedish forest landscapes: at the south site a mesic to dry stone-rich shallow sandy-silty podzol, at the NE site a mesic clay-rich silty podzolized deep glacial till with low stone abundance and at the NW site a dry to mesic silty-sandy podzolized deep glacial till with moderate-high stone abundance (Mantel et al. 2023; SLU 2023). As well as many other environmental differences between the sites (Table 1), there were also minor differences in site preparation methods, but all produced isolated planting spots. At all sites, harvest and site preparation occurred in the same year as trial establishment. At the south (S) site, the aim was to make inverted planting spots, but shallow soil and high stoniness compromised the outcome, leaving patches of disturbed soil rather than true inversion. At the northwest (NW) site the soil conditions allowed for true inverted spots. At the northeast (NE) site an alternative method was used, with a milling aggregate producing a depression with mixed soil material in small mounds on each side of the depression. The reason behind the choice of method on this site was in part related to the site conditions with a fine mesic soil and partly flat areas. In fine mesic soil with high clay content the risk of water logging is relatively high. Water logging is less likely on elevated spots, which are not produced at inversion; this method was not therefore considered appropriate at the NE site.
Each prepared spot was planted with one (S, NW) or two (NE) seedlings. Seedlings were marked up with sticks to aid the identification of those to be included in the trial. For the NE site, only one of the two seedlings planted at each prepared spot was chosen to be included in the trial first at the time of the first inventory. We planted two seedlings at each spot at this site because of the soil conditions. The fine mesic soil presents a high risk of frost heaving, which can cause newly planted seedlings’ root substrate being pushed up from the ground. Hence, two seedlings at each spot safe-guarded against a high mortality from frost heaving. Only spots where both seedlings had died became classified as ‘dead’. At spots where only one seedling had died, the living one was chosen; where both seedlings survived, the healthiest looking one was chosen. At the S site the plots were randomly distributed and scattered over a large area, some being adjacent and some isolated. These plots were located on relatively flat parts of an otherwise hummocky harvested landscape. At the NE and NW sites, all the treated plots were placed adjacent to each other in one area, and all the untreated plots adjacent to each other next to the treated plots, i.e., within the same harvested area but not randomly positioned (maps of the three sites in Supplementary file S1). Thus, within-site differences in the landscape could interfere with the effect of AP. However, since there was variation in slope both within and between plots on both sites and in both treatments, we assumed most within-site variation to be equally represented in both treated and untreated plots. Lastly, another difference between sites was that at the two northern sites a second dose of AP was added at the base of each treated seedling in the following year, by manually dropping one dose of AP with an arGrow® dispenser at the base of each seedling.
Differences between sites in outcome for survival, seedling growth, and degree of damage for the different species is naturally expected due to both the environmental differences as well as differences in methods and trial setup between the sites. This makes it hard to disentangle causality behind differences in performance between sites. However, since analysis of causality behind site differences would be doubtful with only three sites anyway, it does not form a part of this study. Thus, in the present study these preliminary data are analyzed for each site as separate case studies.
2.2 Data collection
Survival and growth of the seedlings were recorded following the second growing season in the field for all sites, the south site early December 2021, the northeast site early September 2021 and the northwest site in the middle of October 2020. Seedling survival was defined as the number of seedlings that were not dead or missing. The missing seedlings were detected by matching the inventory against the number of seedlings in a survival inventory made following the first year in field. At the NW site, one row in block 6, two rows in block 10 and one row in block 12 were missed in the second season’s inventory; these rows were therefore removed from analyses to avoid creating a bias towards lower survival. Vital seedlings were defined as those in vitality class 1 (no, or minor damage recorded). The cause of damage was registered when such was identifiable.
Seedling growth in all three species was quantified by seedling height and stem base diameter. Only measurements of seedlings defined as vital were used in the analyses. The analyses of growth data should therefore be seen as potential growth given that no damage affected the seedlings. Number of measured seedlings in each plot differed between 0(2 plots) and 72 due to: 1) different numbers of seedlings planted because of the species mix used, and site because of different plant density; 2) difference in number of available vital seedlings to measure depending on both site and species; 3) differences in inventory protocols between sites – for the NW site, only every second available vital seedling was measured. For details of alive and vital seedlings per plot, see Suppl. file S2.
Seedling damage at the south site was identified visually according to a vitality class scale from 0–6 (0 = no visible damage, 1 = minor damage, i.e., visible damage that was not severe enough to cause any visual decrease in vitality of the seedling, 2 = medium damage, i.e., some appearance of decrease in vitality of the seedling, 3 = severe damage, i.e., the seedling has clearly visible decrease in vitality, 4 = lethal damage, i.e., the seedling do not appear to be able to survive, 5 = dead and 6 = missing or registered as dead already at the time of the previous inventory). Cause of damage was registered when this was possible to determine visually, including the categories fungi, frost, drought, oxygen deficiency, vegetative competition, browsing (i.e., when branches and/or leaves are clearly removed by a large herbivore, including bitemarks where a branch is removed and stripped branches), pine weevil (i.e., small but deep gnaw-marks on the stem characteristic for pine weevil), other insects or other/unknown. Damage recording at the north sites was less detailed. Seedlings were classified as undamaged when no visible damage occurred, damaged when the seedling displayed visible damage, or as dead. Damage type was registered, such as multiple stems, bent, dry top, broken top etc. Damage cause was registered when this was possible to determine, such as browsing, water logging and drought. For an overview of seedling status including dead, damaged and vital seedlings, see Suppl. file S3.
With only one plot of each species mix for each AP-treatment at each site, potential species mix effects on damage were not suitable for statistical analysis at site level. Regarding browsing, the lack of replicates increases the risk that any given plot might be one through which ungulates move more frequently due to the position in the landscape that might affect the ungulates roaming patterns (e.g., distance to forest, roads, water, good feeding areas etc.), regardless of the species mix. We did not therefore attempt to find out whether species composition had any effect on browsing. However, pine weevil can be assumed to be present in all plots due to their attraction to fresh stumps regardless of other site features. To get an indication of whether species composition had any effect on susceptibility to pine weevil damage, we therefore quantified the levels of damage in the different species mixes in relation to AP-treatment at the S site.
2.3 Statistical analyses
R-studio was used for all analyses (R Core Team 2021). Each site was analyzed separately. Generalized mixed effect models were constructed for survival and damage by specific agents respectively using the glmer function in the [lme4] package (Bates et al. 2015), with binomial distribution as the response variables, where 1 = alive, 0= dead for the analyses of survival and 1 = damaged by specific agent, 0= not damaged by specific agent for the analyses of damage. Linear mixed models were constructed for height and stem base diameter using the lmer function in the [lmerTest] package (Kuznetsova et al. 2017). Only measurements of seedlings classified as vital were used in the analyses. AP-treatment and species were set as fixed factors and species composition nested within species was set as a random factor. The random factor was set to account both for and the varying numbers of seedlings between the different species compositions, and other potential within-site variability.
Each model was tested for interactions between treatment and species with ANOVA type III using the [car] package (Fox and Weisberg 2018). In cases where there was no significant interaction, the interaction term was dropped from the model and the reduce model was tested again with ANOVA type II using the [car] package. The [emmeans] package (Lenth 2021) was used to produce estimated marginal means from the models and the [ggplot2] package (Wickham 2016) was used to present results graphically.
3 Results
3.1 The south site
At the S site, survival rates of spruce and birch seedlings following two vegetative growth periods were generally higher than that of pine (Fig. 1a). For the pine seedlings, AP-treatment increased the survival rate: 60% of the AP-treated pine seedlings had survived compared to 28% of the non-treated control seedlings (Fig. 1a, Table 2). According to the measurements of seedling height and stem base diameter used as proxies for seedling growth, AP-treatment had no effect on growth. Birch seedlings grew more than pine and spruce seedlings regardless of treatment (Fig. 1b-c, Table 2). The main damage suffered by seedlings that had survived two vegetative growth periods was from pine weevil, followed by ungulate browsing. Pine weevil damage was frequent on the conifer seedlings with more than 50% of both pine and spruce seedlings being damaged by them, while less than 25% of the birch seedlings showed any signs of pine weevil damage (Fig. 2, Table 3). For the pine seedlings, a significantly higher proportion of the AP-treated seedlings (79%) than the control seedlings (52%) were damaged (Fig. 2, Table 2). For the birch seedlings, a significantly lower proportion of the AP-treated seedlings (2%) than the control seedlings (12%) were damaged. On birch seedlings, browsing damage was very common with >70% of the seedlings having been browsed, while the proportion of browsed seedlings among both pine and spruce was always <25% (Fig. 2, Table 3). For both birch and spruce the same pattern occurred as seen for the pine weevil damage on pine seedlings, with a higher portion of the AP-treated than the control seedlings damaged by browsing (90% vs 72% for birch and 22% vs 7% for spruce) (Fig. 2, Table 3).
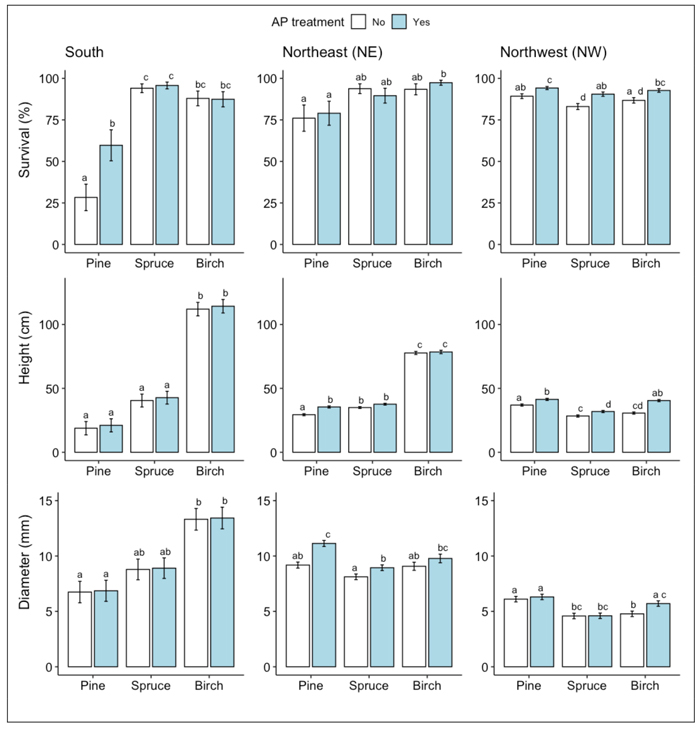
Fig. 1. Results from ANOVA analysis for A) survival, B) height, and C) stem base diameter means of Scots pine, Norway spruce and silver birch at each site respectively following the second field season. Columns indicate percentage survival and error bars indicate standard error (data backtransformed from log scale in survival models). Different letters indicate significant differences. For height and stem base diameter, only measurements of seedlings classified as vital are included.
| Table 2. ANOVA (Type II/III Wald chi-square tests) on generalized mixed linear models for probability of survival and linear mixed models for height and stem base diameter for each site following the second growing season in the field. Significant results are highlighted in bold. | |||||||||
| South (S) | Northeast (NE) | Northwest (NW) | |||||||
| Survival | X2 | df | Pr(>X2) | X2 | df | Pr(>X2) | X2 | df | Pr(>X2) |
| AP | 8.26 | 1 | 0.004 | 0.99 | 1 | 0.319 | 19.19 | 1 | <0.001 |
| Species | 34.57 | 2 | <0.001 | 8.40 | 2 | 0.015 | 8.79 | 2 | 0.012 |
| AP × Species | 13.44 | 2 | 0.001 | 8.92 | 2 | 0.012 | - | - | - |
| Height | |||||||||
| AP | 2.55 | 1 | 0.1103 | 17.12 | 1 | <0.001 | 137.51 | 1 | <0.001 |
| Species | 177.43 | 2 | <0.001 | 2089.17 | 2 | <0.001 | 80.16 | 2 | <0.001 |
| AP × Species | - | - | - | 8.57 | 2 | 0.014 | 30.34 | 2 | <0.001 |
| Diameter | |||||||||
| AP | 0.20 | 1 | 0.65 | 44.94 | 1 | <0.001 | 25.55 | 1 | <0.001 |
| Species | 24.46 | 2 | <0.001 | 23.81 | 2 | <0.001 | 23.14 | 2 | <0.001 |
| AP × Species | - | - | - | 13.80 | 2 | 0.001 | 27.17 | 2 | <0.001 |
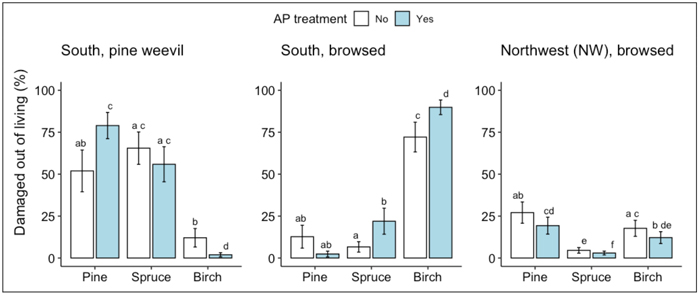
Fig. 2. Results from ANOVA analysis for damage to living seedlings of Scots pine, Norway spruce and silver birch by A) pine weevil and B) browsing at the south site, and C) browsing at the NW site following the second field season. Columns indicate percentage of living seedlings damaged and error bars indicate standard error (data backtransformed from log scale used in models.) Different letters indicate significant differences.
| Table 3. ANOVA (Type II/III Wald chi-square tests) on generalized mixed linear models for probability of registered damage following the second growing season in the field; browsing and pine weevil for the south site and browsing for the northwest site. Significant results are highlighted in bold. | |||||||||
| South, pine weevil | South, browsing | Northwest, browsing | |||||||
| Damage | X2 | df | Pr(>X2) | X2 | df | Pr(>X2) | X2 | df | Pr(>X2) |
| AP | 2.81 | 1 | 0.094 | 0.95 | 1 | 0.331 | 9.70 | 1 | 0.002 |
| Species | 32.29 | 2 | <0.001 | 47.52 | 2 | <0.001 | 17.84 | 2 | <0.001 |
| AP × Species | 27.74 | 2 | <0.001 | 17.22 | 2 | <0.001 | - | - | - |
For pine, the AP-treatment effect on pine weevil damage levels was consistent for all different species mixes, <60% of the living seedlings were damaged by pine weevil in all untreated plots and >60% in all AP-treated plots. For spruce, we measured more pine weevil damage (>70% of living seedlings) when it was mixed with birch, than when alone or mixed with pine (<60% of living seedlings) independent of AP-treatment, while spruce damage in the three-species mix was intermediate. Pine weevils only damaged birch seedlings planted as single species stands (17% in the untreated plot and 7% in AP-treated plot) or when planted with pine in the untreated plot (43%).
3.2 The northeast site
At the NE site, survival rates following two vegetative periods were generally higher for birch than pine seedlings (>90% vs <80% for the two species, respectively) while survival of spruce seedlings was intermediate (Fig. 1a, Table 2). The difference in survival was, however, only significant between AP-treated birch vs pine, there being no significant positive effect of AP-treatment on survival for either species individually (Fig. 1a, Table 2). Concerning growth, birch grew more than twice as tall as the other species, regardless of treatment (Fig. 1b-c, Table 2). AP-treatment had a significant positive effect on stem base growth of pine and spruce, but not of birch (Fig. 1b-c, Table 2). On average, AP-treated pine was 21% taller and had a stem base diameter that was c. 22% wider than untreated pine, while AP-treated spruce had on average 13% larger stem base diameter than untreated spruce (Fig. 1b-c, Table 2). The main damage at the NE site to seedlings that had survived two vegetative periods was from browsing on birch, and forking, i.e., formation of multiple leader shoots for pine. AP-treatment had no significant effect on either form of damage on birch or pine (~70% of AP-treated and ~65% of untreated birch seedlings were browsed and ~13% of both AP-treated and untreated pine seedlings had multiple leader shoots).
3.3 The northwest site
At the NW site, survival rates of pine seedlings following two vegetative periods were significantly higher (c. 7%) than that of spruce, while survival of birch did not differ from the other species (within the same treatment) (Fig. 1a, Table 2). AP-treatment significantly increased the survival rate of all three species, as higher proportions of AP-treated seedlings had survived than untreated control seedlings (94% vs 89% for pine; 90% vs 83% for spruce; 93% vs 87% for birch) (Fig. 1a, Table 2). The growth of all three species was here more within the same range, as compared to the other sites where birch was growing higher than the other two species (Fig. 1b-c, Table 2). AP-treatment had a significant positive effect on seedling height for all three species (11% for pine, 14% for spruce and 32% for birch), but significantly affected stem base diameter (20%) only for birch (Fig. 1b-c, Table 2). The main damage found on the NW site seedlings was from browsing. The effect of AP-treatment on browsing was significant for all three species: a smaller proportion of the AP-treated seedlings was damaged by browsing than control seedlings (19% vs 27% for pine; 3% vs 5% for spruce; 12% vs 18% for birch) (Fig. 2, Table 3). The lighter browsing damage on spruce compared to pine and birch was significant within each of the two treatments (Fig. 2, Table 3).
4 Discussion
Survival, growth, susceptibility to damage as well as the effect of AP-treatment varied among the three tree species across the three sites, indicating that challenges to the establishment of planted seedlings of all three species depend on site-specific characteristics. The variations in survival and growth between species can be considered a consequence of how well adapted each species is to a particular site and their varying susceptibilities to different agents of damage. Particularly, high mortality in the south suggests the establishment of pine to be a greater challenge in southern than in northern Sweden. One of the reasons for why the establishment of pine is more difficult in this area is that pine weevil pressure is more severe in the south and closer to the coast due to the relatively higher accumulated temperature in these areas compared to northern inland areas of Sweden (Nordlander et al. 2017). In addition, the trees’ chemical defenses against pine weevil are weaker in southern than in northern populations in Sweden (Yazdani et al. 1985; Yazdani and Nilsson 1986). Planting in positions where seedlings are surrounded by exposed mineral soil following site preparation, as done on all sites in this study, reduce pine weevil damage since the weevils tend to avoid open areas (Örlander and Nilsson 1999; Thorsen et al. 2001; Petersson and Örlander 2003; Petersson et al. 2005; Wallertz et al. 2005; Nordlander et al. 2011). A complementary protection by some sort of mechanical barrier is commonly used to further reduce the risk of pine weevil damage in pine weevil dense areas, as was done in this study for the conifers at the south site (Table 1). However, no single method can provide full protection in pine weevil dense areas, and it is important to consider any measures that may reduce pine weevil damage further, including enhanced establishment and early growth to overcome the greatest risks of lethal damage.
Our hypothesis of AP-treatment increasing survival and growth of all three species across all three sites was only partly corroborated. While AP-treatment had a positive effect on survival of all three species at the NW site, this was the case only with pine at the S site. At the NE site none of the three tree species showed any significant effect by AP-treatment on survival. It should be noted that the approach of planting two seedlings at each spot at the NE site and choosing the surviving/most vital seedling to be included in the trial created a bias towards higher survival that may have compromised the results for survival at the this site, perhaps making any difference between AP-treated and untreated seedlings less pronounced than they may have been if there was only one seedling planted per spot.
Among the undamaged trees, birch outgrew pine and spruce on both the S and NE sites. This can pose a challenge when the trees grow big enough to interfere with each other’s growth space if the purpose is to establish a mixed tree species stand. At the NE-site, the AP-treatment however increased the growth rate of the conifers, but not to the extent that they grew as tall as birch. At the NW site, growth was more equal between species and the AP-treated seedlings of all three species showed both higher survival and growth than the untreated seedlings. This observed pattern of birch being less height dominant with a shorter growing season (the NW site has shorter growing season than the NE site despite similar latitude due to its inland location, see Table 1) could be a natural consequence of birch having a longer duration of height growth as well as responding more strongly to increased temperature than either pine or spruce (Nissinen et al. 2020; Pikkarainen et al. 2022). By contrast, the benefit of AP-treatment for birch was highest at the NW site where there was a positive effect on both survival and growth. Unexpectedly, the height pattern of pine was reversed, it being lower at southern than northern sites. We suspect that the unexpectedly low growth of pine in the S site in comparison to the northern sites might be due to pine weevil damage, which not only affect survival negatively but also hampers growth (Örlander and Nilsson 1999). The positive effects of AP-treatment on pine survival and growth are in line with earlier findings, except for the lack of a positive effect on growth at the S site. Previous research found an increasing positive effect on growth induced by AP-treatment with a longer growing season (Häggström et al. 2021). However, in contrast to the present study, Häggström et al. (2021) did not include the most southern part of Sweden where pests and vegetation competition have a greater effect, possibly overshadowing any positive effect on growth the AP-treatment might have had.
Our hypothesis regarding increased vitality to withstand damage for seedlings treated with AP was also only in part corroborated. Pine survival at the S site was improved by the AP-treatment, despite that the attack rate from pine weevils was significantly higher on the treated than the untreated seedlings. Cause of mortality was generally unknown, but a high proportion of the remaining living seedlings were damaged by pine weevil, so it seems likely that they were responsible for the high mortality rate at the S site. Assuming that pine weevils was equally present in all plots due to their attraction to fresh tree stumps regardless of other site features, the pine weevil damage pressure was likely equal for untreated and AP-treated seedlings. Thus, we interpret the results such as that the pine weevil damage caused a lethal outcome for a larger proportion of the untreated pine seedlings, while leaving a larger number of AP-treated seedlings that remained alive with pine weevil damage. The mechanism behind the enhanced survival of pine seedlings treated with AP may be related to these seedlings being more vital and thereby more resistant to pine weevil damage than untreated seedlings. This finding could potentially have been related to a better growth rate of AP-treated seedlings, since seedlings with a wider stem base are less likely to be girdled by pine weevils (Örlander and Nilsson 1999; Wallertz et al. 2005). However, at the S site neither AP-treated nor untreated seedlings had reached a stem base diameter threshold (10–12 mm) that confers decrease in lethal outcome of pine-weevil damage (Thorsen et al. 2001; Wallertz et al. 2005). Furthermore, the difference in growth between AP-treated and untreated seedlings was not significant, so this mechanical defense is not likely to be the explanation in this case. Another explanation could potentially be that AP-treated seedlings were better able to withstand pine weevil damage due to better water and nutritional status. Though this was not expressed in better above-ground growth, these resources are needed for defense mechanisms as well.
Insects that chew on bark will have to deal with a tree’s chemical defenses such as toxic phenols, and terpenoids in resin that act both to kill intruding insects and heal wounds (Trapp and Croteau 2001; Bonello et al. 2006). The terpenoid α-pinene attracts pine weevil to newly cut trees; another, limonene, acts to inhibit the attractiveness of α-pinene (Nordlander 1990; Danielsson et al. 2008). Water stress decreases the ability of trees to produce both resin and limonene, while it increases α-pinene production (Selander and Immonen 1992). This further emphasises the importance of early root growth allowing good water uptake by seedlings. It could be a contributory factor explaining why the AP-treated seedlings exhibited better survival and a greater proportion of vital seedlings, despite their suffering a high level of pine weevil attack. However, the relation between chemical defense and seedling vigor is not always straight-forward. While some studies suggest that limited nitrogen availability negatively affects plant defenses (Bonello et al. 2006), others have found that defense substances decrease in N-fertilized trees (Witzell and Martín 2008). In our case, AP may have improved seedlings root area and ectomycorrhizal colonization to acquire more resources enabling the seedlings to withstand the damage by pine weevil better than the untreated ones.
Yet another potential explanation as to why a larger share of the surviving AP-treated pine seedlings were attacked by weevil, yet experienced lower mortality than untreated seedlings, might be related to plant phenols, which are derived from the amino acid phenylalanine (Bennett and Wallsgrove 1994). The presence of these antifeedant defense substances do not hinder weevils from initially chewing bark, but they will chew less which should lead to decreased risk of lethal damage (Fedderwitz et al. 2016). In a small nursery pilot project, it was found that pine seedlings grown on AP had higher levels of phenylalanine in their bark compared with those grown on ammonium nitrate (Näsholm T. 2022, unpublished data, Suppl. file S4). This finding suggests that AP-treated seedlings should have better potential to produce feeding deterrents. This requires confirmation through further research, but it could be an important tool to improve seedling survival in areas with high pine weevil feeding pressure.
Since there was only one plot of each species mix for each AP-treatment at each site, species mix effects were not suitable for statistical analysis at site level due to the risk of potential interference from other within-site variables. The three sites will be able to be used as replicates in future analyses of species mix effects under inter- and intra-specific competition. However, in the case of damage, this approach would be hard to justify since seedlings’ susceptibilities will vary both according to the agent of damage and environmental differences between sites. Nevertheless, we quantified and compared the levels of pine weevil damage in different species compositions to get an indication of whether species composition had any effect on susceptibility to pine weevil damage. The pattern of pine weevil feeding on spruce and birch seemed to be affected by species composition of the stands and related to their feeding preferences. Spruce was more frequently attacked when growing with birch than in pure plantings, a pattern that agrees with pine weevil preferring conifers over broad leaved trees (Day et al. 2004; Löf et al. 2004; Månsson and Schlyter 2004). Spruce was also less frequently attacked when planted with pine, as expected since pine weevils prefer pine when both conifers are present (Day et al. 2004; Wallertz et al. 2005). Birch was only attacked when no, or only very few pine seedlings were available, as in the untreated pine-birch plot where only four living pine seedlings remained, i.e., there was not much else for the pine weevils to feed on other than birch in this plot. Our interpretation of this pattern is that the substantially higher attack rate in the untreated pine-birch plot (43%) compared with the pure birch plots (17% in the untreated and 7% in the treated) might be due to more pine weevil initially being attracted to the plot where pine seedlings were present. As their preferred food source, the pine in the pine-birch plots suffered higher pine weevil pressure per seedling than occurred in the other mixtures. When most of the pine seedlings eventually died in the untreated plot, the remaining pine weevils transferred to the birch seedlings as their food source.
The effect of AP-treatment on browsing was contradictory. At the S site, a large proportion of the birch was browsed upon, and for both birch and spruce more so in AP-treated than in control plots. This would be expected, since fertilization may increase N-content in the seedlings, giving them a higher nutritional value and thus attract browsing herbivores (Månsson et al. 2009; Burney and Jacobs 2011). By contrast, at the NW site the AP-treated trees of all three species were less browsed upon than the untreated seedlings. The opposing results could be due to many reasons. For example, fertilization increases not only nitrogen content but also the levels of chemical defense substances in the trees, which may alter feeding preferences for ungulates (Burney and Jacobs 2011). The different proveniences representing the different sites may have responded in different ways to the fertilization. However, the simplest explanation could be that the positioning of the untreated plots may have happened to be where moose move through more frequently. For example, at the NW site, all AP-treated plots were in the part of the clear-cut which was closer to a road than the untreated plots, and which might therefore affect their browsing behavior if the moose avoid being close to the road. However, any true effect of less browsing by moose on AP-treated seedlings, could be an important tool when regenerating pine. Further research on this topic is required to clarify the issue.
The most common damage registered on pine at the NE site was forking, i.e., multiple leader shoots. Multiple leader shoot can be an effect of previous browsing. However, browsing alone does not account for all occurrences of multiple stems. Another potential pre-cause of multiple leader shoots can be the occurrence of summer shoots (i.e., new shoots already developing during the summer on current year´s buds, instead of the regular two-year process). Summer shoots, termed ‘proleptic shoots’ that grow from the lateral buds at the base of the current year’s terminal bud, can result in a temporary inhibition of apical dominance, which in turn sometimes leads to multiple leader shoots, i.e., forking (Aldén 1971). In southern Sweden the occurrence of summer shoots and multiple stems has increased in recent years, both in planted and naturally regenerated pine seedlings, but the cause is not yet fully understood (Högberg et al. 2021). However, Aldén (1971) found a positive correlation with growth-promoting variables such as increased CO2, optimal moisture level, as well as nutrient conditions changing from poor to optimal, indicating that increased occurrence of multiple leader shoots might be related to changes in environmental conditions. More recent research also indicates that insufficient chilling time during dormancy of many tree species in temperate and boreal areas can delay budburst, which can lead to growth abnormalities including terminal shoots being shorter than laterals in conifers (Laube et al. 2014; Harrington and Gould 2015; Man et al. 2017). Anyhow, there was no effect of AP-treatment on the number of multiple leader shoots at the NE site, which neither confirms nor contradicts any potential theory regarding the occurrence of this phenomenon.
Our study has demonstrated the effects of AP-treatment to be complex and site specific, i.e., the same results cannot be expected at all sites. Nevertheless, the positive effect on pine seedling survival following AP-treatment appears promising and may improve pine regeneration, especially in the southern part of Sweden. The positive effect on the growth of conifers at the northern sites might present a tool to decrease the time of small seedlings being at risk for damage by insects and herbivores. However, further experimental work is needed to confirm whether the same positive effect of AP-treatment on pine seedling survival in south Sweden and growth in north Sweden might also be observed beyond these specific sites. Further, only one seedling type for each species was used as each site in this study which may have affected the results, since AP-treatment response might potentially differ between seed sources due to genetic variation in nitrogen use efficiency (Li et al. 1991). We therefore recommend future studies to include several different sources of seedling material at each site to evaluate the potential genetic dependent response. Whether the positive effects of AP-treatment are due to the repeated addition of arginine phosphate (as done at the northern sites), or if a single addition (as done at the southern site) would have been sufficient to reach the same result we cannot tell from this study. However, studying the long-term effects on these and other sites, where AP-treatments were only applied at planting, will allow further comparisons to be made. If the effect of a single treatment seems to fade after some time, while repeated treatments continue to give an increased effect, we could assume that repeated additions of AP would be the better option. To be able to compare these effects more exactly, we suggest a trial in which control, single, and repeated additions of AP are tested at the same sites.
5 Conclusion
The effects of AP-treatment on survival, growth and levels of damage varied between the three sites. At the S site, AP-treatment increased survival of pine. A higher share of the surviving seedlings was attacked by pine weevil when AP-treated than untreated, indicating that the increased survival of AP-treated seedlings could be related to increased tolerance against pine weevil damage. More AP-treated than untreated birch and spruce were also browsed at the S site, while there was no AP-treatment effect on growth for any of the three species. On the NE site, there was no effect on survival or level of damage, but a positive effect on stem base growth for pine and spruce. At the NW site, AP-treatment had a positive effect on survival and growth for all three species which were also less browsed when AP-treated than untreated. Birch grew taller than the conifers at the S and NE site, while pine and birch grew more equally at the NW site. The use of AP-treatment appears overall to be more useful regarding effects on growth and survival on the NW site. However, the effects on the level of damage from pine weevil vs survival for pine at the S site indicates that AP-treatment might make pine less vulnerable to lethal pine-weevil damage, which is a topic for further research. In practical forestry, AP-treatment could be a potential tool to increase survival of planted pine in the southern parts of Sweden and to increase early growth after planting of conifers in the northern parts of Sweden to increase the chance of successful establishment of a newly regenerated forest stand.
Declaration of openness of research materials, data, and code
Data files and code for statistics and figures in main manuscript is publicly available at the Swedish National Data Service: Häggström B. (2022). Effect of arginine-phosphate addition on early survival and growth of Scots pine, Norway spruce and silver birch. Swedish National Data Service. Version 1. Swedish University of Agricultural Sciences. https://doi.org/10.5878/p1vn-df79.
Authors’ contributions
TL, RL and FS contributed to the conception of the research question and design of the work, BH contributed with acquisition, analysis, and interpretation of data and results as well as scientific writing of the work, AN contributed with scientific writing and AN, RL, TL and FS contributed with revising it critically for sound and intellectual content.
Acknowledgements
We thank Matej Domevscik, Simon Bailly and Dorothea Zannantonio for inventory assistance at the northern sites, and Mikael Andersson at the Asa Experimental Forest and Research Station for providing data from the experiment at the south site. We also thank the landowners, Sveaskog (S and NW sites) and T. Lundmark (NE site) for the provision of areas to set up the experiments.
Funding
This work was supported by the Knut and Alice Wallenberg Foundation under Grant number KAW 2016.0341, KAW 2018.0259, the Swedish Governmental Agency for Innovation Systems under Grant number 2016-00504; and by Estonian Research Council grant PSG730. The funding of BH’s PhD position was provided by from the Research School in Forest Genetics, Biotechnology and Breeding at the Umeå Plant Science Centre, UPSC, at the Swedish University of Agricultural Sciences (SLU) being part of the Competence Centre program of the Swedish Governmental Agency for Innovation Systems (VINNOVA). Funding of the field study was provided by the Trees and Crops for Future program at SLU and the Knut and Alice Wallenberg Foundation.
References
Aldén T (1971) Influence of CO2, moisture and nutrients on the formation of Lammas growth and prolepsis in seedlings of Pinus silvestris L. Stud For Suec 93, article id 5772. http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-9-105.
Bates D, Machler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. https://doi.org/10.18637/jss.v067.i01.
Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defense-mechanisms. New Phytol 127: 617–633. https://doi.org/10.1111/j.1469-8137.1994.tb02968.x.
Bergqvist J (2017) Hjortvilt. [Cervids] In: Witzell (ed) Skogsskötselserien nr 12, Skador på skog del 1. [Forest management series no 12, Damage on forest part 1]. Skogsstyrelsen, pp 88–94.
Bhatti JS, Apps MJ, Lal R (2006) Anthropogenic changes and the global carbon cycle. In: Bhatti JS, Lal R, Apps MJ, Price MA (eds) Vol. Part II: Managed ecosystems – state of knowledge. Section 4. CRC Press, Taylor & Francis Group, Boca Raton, Florida, USA. Clim Chang Manage Ecosyst, pp 69–92.
Bonello P, Gordon TR, Herms DA, Wood DL, Erbilgin N (2006) Nature and ecological implications of pathogen-induced systemic resistance in conifers: a novel hypothesis. Physiol Mol Plant Pathol 68: 95–104. https://doi.org/10.1016/j.pmpp.2006.12.002.
Brand DG (1991) The establishment of Boreal and Sub-boreal conifer plantations – an integrated analysis of environmental-conditions and seedling growth. For Sci 37: 68–100. https://doi.org/10.1093/forsci/37.1.68.
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63: 625–644. https://doi.org/10.1051/forest:2006042.
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6, article id 547. https://doi.org/10.3389/fpls.2015.00547.
Burdett AN (1990) Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can J For Res 20: 415–427. https://doi.org/10.1139/x90-059.
Burney OT, Jacobs DF (2011) Ungulate herbivory of regenerating conifers in relation to foliar nutrition and terpenoid production. For Ecol Manage 262: 1834–1845. https://doi.org/10.1016/j.foreco.2011.07.035.
Castro D, Schneider AN, Holmlund M, Nasholm T, Street NR, Hurry V (2021) Effects of early, small-scale nitrogen addition on germination and early growth of Scots pine (Pinus sylvestris) seedlings and on the recruitment of the root-associated fungal community. Forests 12, article id 1589. https://doi.org/10.3390/f12111589.
Dahlgren Lidman F (2022) Natural regeneration and management of birch. Univ agric Suec 2022:54. https://res.slu.se/id/publ/118906.
Danell K, Huss-Danell K and Bergstrom R (1985) Interactions between browsing moose and two species of birch in Sweden. Ecology 66: 1867–1878. https://doi.org/10.2307/2937382.
Danielsson M, Kannaste A, Lindstrom A, Hellqvist C, Stattin E, Langstrom B, Borg-Karlson AK (2008) Mini-seedlings of Picea abies are less attacked by Hylobius abietis than conventional ones: is plant chemistry the explanation? Scand J For Res 23: 299–306. https://doi.org/10.1080/02827580802203560.
Day K, Nordlander G, Kenis M, Halldorson G (2004) General biology and life cycles of bark weevils. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, pp 331–349. https://doi.org/10.1007/978-1-4020-2241-8_14.
Domevščik M, Häggstrom B, Lim H, Öhlund J, Nordin A (2023) Large-scale assessment of artificially coated seeds for forest regeneration across Sweden. New For 54: 255–267. https://doi.org/10.1007/s11056-022-09920-2.
Fedderwitz F, Nordlander G, Ninkovic V, Björklund N (2016) Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. J Pest Sci 89: 97–105. https://doi.org/10.1007/s10340-015-0684-9.
Felton AM, Holmström E, Malmsten J, Felton A, Cromsigt JPGM, Edenius L,Ericsson G, Widemo F, Wam HK (2020) Varied diets, including broadleaved forage, are important for a large herbivore species inhabiting highly modified landscapes. Sci Rep 10, article id 1904. https://doi.org/10.1038/s41598-020-58673-5.
Fox J, Weisberg S (2018) An R companion to applied regression. Sage publications. https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Gill RMA (1992) A review of damage by mammals in north temperate forests: 1. Deer. Forestry 65: 145–169. https://doi.org/10.1093/forestry/65.2.145.
Grossnickle SC (2000) Ecophysiology of northern spruce species: the performance of planted seedlings. NRC Res Press. http://www.nrcresearchpress.com/doi/book/10.1139/9780660179599#.U16Tm7lOXIU.
Grossnickle SC (2005) Importance of root growth in overcoming planting stress. New For 30: 273–294. https://doi.org/10.1007/s11056-004-8303-2.
Gruffman L, Ishida T, Nordin A, Näsholm T (2012) Cultivation of Norway spruce and Scots pine on organic nitrogen improves seedling morphology and field performance. For Ecol Manage 276: 118–124. https://doi.org/10.1016/j.foreco.2012.03.030.
Gruffman L, Palmroth S, Näsholm T (2013) Organic nitrogen uptake of Scots pine seedlings is independent of current carbohydrate supply. Tree Physiol 33: 590–600. https://doi.org/10.1093/treephys/tpt041.
Häggström B, Domevščik M, Öhlund J, Nordin A (2021) Survival and growth of Scots pine (Pinus sylvestris) seedlings in north Sweden: effects of planting position and arginine phosphate addition. Scand J For Res 36: 423–433. https://doi.org/10.1080/02827581.2021.1957999.
Harrington CA, Gould PJ (2015) Tradeoffs between chilling and forcing in satisfying dormancy requirements for Pacific Northwest tree species. Front Plant Sci 6, article id 120. https://doi.org/10.3389/fpls.2015.00120.
Hedwall P-O, Gruffman L, Ishida T, From F, Lundmark T, Näsholm T, Nordin A (2018) Interplay between N- form and N-dose influences ecosystem effects of N addition to boreal forest. Plant Soil 423: 385–395. https://doi.org/10.1007/s11104-017-3444-1.
Heräjärvi H (2001) Technical properties of mature birch (Betula pendula and B. pubescens) for saw milling. Silva Fenn 35: 469–485. https://doi.org/10.14214/sf.581.
Högberg K-A, Nilsson O, Palmér S (2021) Tillväxtstörningar på tallplantor. [Growth disturbances in pine seedlings]. Arbetsrapport 1092–2021, Skogforsk.
Inselsbacher E, Ohlund J, Jämtgård S, Huss-Danell K, Näsholm T (2011) The potential of microdialysis to monitor organic and inorganic nitrogen compounds in soil. Soil Biol Biochem 43: 1321–1332. https://doi.org/10.1016/j.soilbio.2011.03.003.
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82: 1–26. https://doi.org/10.18637/jss.v082.i13.
Laube J, Sparks TH, Estrella N, Hofler J, Ankerst DP, Menzel A (2014) Chilling outweighs photoperiod in preventing precocious spring development. Glob Chang Biol 20: 170–182. https://doi.org/10.1111/gcb.12360.
Lenth R (2021) Emmeans: estimated marginal means, aka least-squares means. R package version 1.7.1-1. https://CRAN.R-project.org/package=emmeans.
Li B, McKeand SE, Allen HL (1991) Genetic variation in nitrogen use efficiency of loblolly pine seedlings. For Sci 37: 613–626. https://doi.org/10.1093/forestscience/37.2.613.
Löf M, Isacsson G, Rydberg D, Welander TN (2004) Herbivory by the pine weevil (Hylobius abietis L.) and short-snouted weevils (Strophosoma melanogrammum Forst. and Otiorhynchus scaber L.) during the conversion of a wind-thrown Norway spruce forest into a mixed-species plantation. For Ecol Manage 190: 281–290. https://doi.org/10.1016/j.foreco.2003.10.027.
Man R, Lu P, Dang Q-L (2017) Insufficient chilling effects vary among boreal tree species and chilling duration. Front Plant Sci 8, article id 1354. https://doi.org/10.3389/fpls.2017.01354.
Månsson PE, Schlyter F (2004) Hylobius pine weevils adult host selection and antifeedants: feeding behaviour on host and non-host woody scandinavian plants. Agric For Entomol 6: 165–171. https://doi.org/10.1111/j.1461-9563.2004.00217.x.
Månsson J, Bergström R, Danell K (2009) Fertilization – effects on deciduous tree growth and browsing by moose. For Ecol Manage 258: 2450–2455. https://doi.org/10.1016/j.foreco.2009.08.025.
Mantel S, Dondeyne S, Deckers S (2023) World reference base for soil resources (WRB). Earth Systems and Environmental Sciences, Elsevier. https://doi.org/10.1016/B978-0-12-822974-3.00161-0.
Niemelä P, Danell K (1988) Comparison of moose browsing on Scots pine (Pinus sylvestris) and lodgepole pine (P. contorta). J Appl Ecol 25: 761–775. https://doi.org/10.2307/2403744.
Nissinen K, Virjamo V, Kilpelainen A, Ikonen VP, Pikkarainen L, Arvas IL, Kirsikka-aho S, Peltonen A, Sobuj N, Sivadasan U, Zhou X, Ge ZM, Salminen T, Julkunen-Tiitto R, Peltola H (2020) Growth responses of boreal Scots pine, Norway spruce and silver birch seedlings to simulated climate warming over three growing seasons in a controlled field experiment. Forests 11, article id 943. https://doi.org/10.3390/f11090943.
Nordin A, Uggla C and Nasholm T (2001) Nitrogen forms in bark, wood and foliage of nitrogen-fertilized Pinus sylvestris. Tree Physiol 21: 59–64. https://doi.org/10.1093/treephys/21.1.59.
Nordlander G (1990) Limonene inhibits attraction to α-pinene in the pine weevils Hylobius abietis and H. pinastri. J Chem Ecol 16: 1307–1320. https://doi.org/10.1007/BF01021028.
Nordlander G, Hellqvist C, Johansson K, Nordenhem H (2011) Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. For Ecol Manage 262: 2354–2363. https://doi.org/10.1016/j.foreco.2011.08.033.
Nordlander G, Mason EG, Hjelm K, Nordenhem H, Hellqvist C (2017) Influence of climate and forest management on damage risk by the pine weevil Hylobius abietis in northern Sweden. Silva Fenn 51, article id 7751. https://doi.org/10.14214/sf.7751.
Öhlund J, Näsholm T (2001) Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol 21: 1319–1326. https://doi.org/10.1093/treephys/21.18.1319.
Örlander G, Nilsson U (1999) Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scand J For Res 14: 341–354. https://doi.org/10.1080/02827589950152665.
Örlander G, Gemmel P, Hunt J (1990) Site preparation: a Swedish overview. BC Ministry of Forests.
Petersson M, Örlander G (2003) Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can J For Res 33: 64–73. https://doi.org/10.1139/X02-156.
Petersson M, Örlander G, Nordlander G (2005) Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry 78: 83–92. https://doi.org/10.1093/forestry/cpi008.
Pfeffer SE, Dressel S, Wallgren M, Bergquist J, Kalén C (2022) Browsing damage on Scots pine: direct and indirect effects of landscape characteristics, moose and deer populations. Diversity 14, article id 734. https://doi.org/10.3390/d14090734.
Pikkarainen L, Nissinen K, Ghimire RP, Kivimäenpää M, Ikonen V-P, Kilpeläinen A, Virjamo V, Yu H, Kirsikka-Aho S, Salminen T (2022) Responses in growth and emissions of biogenic volatile organic compounds in Scots pine, Norway spruce and silver birch seedlings to different warming treatments in a controlled field experiment. Sci Total Environ 821, article id 153277. https://doi.org/10.1016/j.scitotenv.2022.153277.
R Core Team (2021) R: a language and environment for statistical computing. https://www.R-project.org/.
Rytter L, Karlsson A., Karlsson M, Stener L-G (2014) Skötsel av björk, al och asp. [Managment of birch, alder and aspen]. Skogsskötselserien nr 9.
Selander J, Immonen A (1992) Effect of fertilization and watering of Scots pine seedlings on the feeding preference of the pine weevil (Hylobius abietis L.). Silva Fenn 26: 75–84. https://doi.org/10.14214/sf.a15637.
Skogsdata (2021) Skogsdata 2021: aktuella uppgifter om de svenska skogarna från SLU Riksskogstaxeringen. [Forest statistics 2021: current data about the Swedish forests, from SLU, The Swedish national forest inventory]. Umeå, Sweden.
SLU (2023) Jordart. [Soil type]. Swedish University of Agricultural Sciences. https://www.slu.se/institutioner/mark-miljo/miljoanalys/markinfo/markprofil/jordart/. Accessed 8 March 2023.
SMHI (2022a) Swedish Meteorological and Hydrological Institute. https://www.smhi.se/pd/klimat/time_period_maps/normal/Nbd_Periodnormal/Nbd_Periodnormal_1981_2010_ar.png. Accessed 17 February 2022.
SMHI (2022b) https://www.smhi.se/pd/klimat/time_period_maps/normal/Arstider_Periodnormal/Arstider_Periodnormal_1981_2010_veg.png. Accessed 17 February 2022.
SMHI (2022c) https://www.smhi.se/pd/klimat/time_period_maps/normal/Temp_Periodnormal/Temp_Periodnormal_1981_2010_ar.png. Accessed 17 February 2022.
SMHI (2022d) https://www.smhi.se/data/meteorologi/kartor/normal/manadsmedeltemperatur-normal/alla-manader/1991-2020. Accessed 6 October 2022.
Tamm CO (1991) Nitrogen-limited and nitrogen-depleted terrestrial ecosystems: ecological characteristics. In: Tamm CO (ed) Nitrogen in terrestrial ecosystems. Ecological Studies 81, Springer, Berlin, Heidelberg, pp 34–49. https://doi.org/10.1007/978-3-642-75168-4_3.
Thiffault N, Jobidon R (2006) How to shift unproductive Kalmia angustifolia – Rhododendron groenlandicum heath to productive conifer plantation. Can J For Res 36: 2364–2376. https://doi.org/10.1139/X06-090.
Thorsen ÅA, Mattsson S, Weslien J (2001) Influence of stem diameter on the survival and growth of containerized Norway spruce seedlings attacked by pine weevils (Hylobius spp.). Scand J For Res 16: 54–66. https://doi.org/10.1080/028275801300004415.
Trapp S, Croteau R (2001) Defensive resin biosynthesis in conifers. Ann Rev Plant Physiol Plant Mol Biol 52: 689–724. https://doi.org/10.1146/annurev.arplant.52.1.689.
Vehviläinen H, Koricheva J (2006) Moose and vole browsing patterns in experimentally assembled pure and mixed forest stands. Ecography 29: 497–506. https://doi.org/10.1111/j.0906-7590.2006.04457.x.
Wallertz K, Örlander G, Luoranen J (2005) Damage by pine weevil Hylobius abietis to conifer seedlings after shelterwood removal. Scand J For Res 20: 412–420. https://doi.org/10.1080/02827580500306954.
Wallgren M, Bergström R, Bergqvist G, Olsson M (2013) Spatial distribution of browsing and tree damage by moose in young pine forests, with implications for the forest industry. For Ecol Manage 305: 229–238. https://doi.org/10.1016/j.foreco.2013.05.057.
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. https://doi.org/10.1007/978-3-319-24277-4.
Witzell J, Martín JA (2008) Phenolic metabolites in the resistance of northern forest trees to pathogens – past experiences and future prospects. Can J For Res 38: 2711–2727. https://doi.org/10.1139/X08-112.
Yazdani R, Nilsson JE (1986) Cortical monoterpene variation in natural populations of Pinus sylvestris in Sweden. Scan J For Res 1: 85–93. https://doi.org/10.1080/02827588609382403.
Yazdani R, Nilsson JE, Ericsson T (1985) Geographical variation in the relative proportion of monoterpenes in cortical oleoresin of Pinus sylvestris in Sweden. Silvae Genet 34: 201–208.
Total of 73 references.

