Fine root distribution, characteristics and rhizosphere soil properties in a mixed stand of Robinia pseudoacacia and Fraxinus velutina in a saline soil
Du Z.-Y., Wang Q.-H., Xing S.-J., Liu F.-C., Ma B.-Y., Ma H.-L., Liu D.-X. (2013). Fine root distribution, characteristics and rhizosphere soil properties in a mixed stand of Robinia pseudoacacia and Fraxinus velutina in a saline soil. Silva Fennica vol. 47 no. 3 article id 970. https://doi.org/10.14214/sf.970
Abstract
The spatial distribution and characteristics of fine roots (< 2 mm in diameter), and rhizosphere soil properties were studied in a mixed planted forest of black locust (Robinia pseudoacacia L.) and velvet ash (Fraxinus velutina Torr.) 27 years after planting in a coastal saline soil of the Yellow River delta, China. The results of fine root analysis showed that the fine roots of both black locust and velvet ash were mainly distributed in the soil layer at 0–20 cm depth and 50–150 cm from trees. The fine root distribution of both species suggests a strategy of avoiding salinity rather than salt –tolerance. The horizontal spread distance of fine roots of velvet ash was evidently longer than that of black locust. The fine root biomass, specific root length, specific root area, specific root volume and root activity were significantly higher for velvet ash in comparison with black locust. The results of soil analysis showed that rhizosphere soil pH of black locust and velvet ash were significantly lower compared with non-rhizosphere soil. The available N content in rhizosphere soil of black locust was higher than that of velvet ash. However, the contents of soluble salt, organic matter, available P and available K in rhizosphere soil of velvet ash were higher than those of black locust. The above results indicated that the differences between black locust and velvet ash in fine root distribution, characteristics and rhizosphere soil properties were the major reasons for that velvet ash showed stronger acclimation responses than black locust to the coastal saline soil.
Keywords
mixed forest;
fine root;
black locust;
velvet ash;
rhizhosphere soil
-
Du,
Shandong Academy of Forestry, 42 Wenhua East Road, Jinan 250014, P. R. China
E-mail
zydu@qq.com

- Wang, Shandong Academy of Forestry, Jinan, P. R. China E-mail wqh0228@163.com
- Xing, Shandong Academy of Forestry, Jinan, P. R. China E-mail xingsj-126@126.com
- Liu, Shandong Academy of Forestry, Jinan, P. R. China E-mail fchliu@126.com
- Ma, Shandong Academy of Forestry, Jinan, P. R. China E-mail mby777@163.com
- Ma, Shandong Academy of Forestry, Jinan, P. R. China E-mail mahlin@163.com
- Liu, Shandong Academy of Forestry, Jinan, P. R. China E-mail llyldx@163.com
Received 7 February 2013 Accepted 5 July 2013 Published 16 September 2013
Views 170967
Available at https://doi.org/10.14214/sf.970 | Download PDF

1 Introduction
Salinity is one of the most severe abiotic stresses affecting plant growth. It can damage or reduce nearly all functions of the plant (Gu et al. 2012). Approximately 10% of the world’s arable land surface consists of saline or sodic soils and the salinization of soils and waters is indicated as one of the leading processes contributing to a worldwide biological degradation (Francois and Maas 1999). Much of salt-affected land is suitable for plantation forestry (Singh 1975) and can be used to help increase supplies of fuel and industrial wood. Tree plantations also provide important contribution in soil and water conservation (Young 1988). However, reports have shown that forest trees are prone to salt stress (Geburek 2000). Sustained and profitable production of forest trees on salt-affected soils is possible if appropriate management decisions are made. One method of improving the productivity of salt-affected land is to plant salt-tolerant tree species.
The Yellow River delta is a region of northeast Shandong province, China, bordering on the Bohai Sea. The region is formed by silt deposition from the Yellow River and supports the extraction of natural resources and other industrial activities, such as crude oil and natural gas prospecting, crude salt extraction, production of chemicals, mechanics, food processing, pulp industry and electricity generation (Zhang and Li 2002). The ecosystems on the Yellow River delta are very fragile and lack resilience against severe soil salinization caused by human activities. Therefore, it is important to restore degraded land by increasing forest cover and improving soil condition. In 1980, the Shandong provincial government initiated a forestation project aimed at protecting the environment and ameliorating soil conditions over a 20-year time span. Since then, a lot of pure or mixed species forests were established with some salt-tolerant tree species including black locust (Robinia pseudoacacia L.), velvet ash (Fraxinus velutina Torr.), Siberian elm (Ulmus pumila L.) and Tree-of-Heaven (Ailanthus altissima (Mill.) Swingle).
Black locust is one of the fastest-growing broad-leaf tree species in the world. The tree has enormous ecological and economic value in addition to salt-tolerance (Boring and Swank 1984; Mierzwa et al. 2009). Black locust has been planted in the Yellow River delta since the 1980s. At present there are 6000 ha of black locust forest growing as pure or mixed stands, depending on the specific land-use systems, which play an important role for windstorm prevention, soil and water conservation, and climate improvement in the Yellow River delta (Song 2001). However, a large-scale forest dieback caused mainly by soil salinity, has resulted in the degradation of tree productivity and ecological benefits. Because of its fast growth rate and salt stress tolerance, velvet ash is also a promising tree for reforestation in the saline soils and has been widely planted for revegetation in the Yellow River delta. Mixed planted forest of black locust and velvet ash was established on a large scale in the 1980s. They are now standing about 30 years old in the coastal saline soil where differences in these two species under these conditions can be studied.
Roots provide detrital carbon to soil organisms and are an important variable influencing the effectiveness of forest ecosystem in absorbing water and nutrients from soils and improving soil quality (Groffman et al. 1992). Although the biomass of fine roots (diameter less than 2 mm) contributes relatively little to total tree biomass (Norby and Jackson 2000), fine roots are major contributors to carbon inputs because of their rapid turnover. The fine roots fulfill mainly nutritional, metabolic and symbiotic functions. Fine root productivity often exceeds aboveground productivity in forest ecosystems, despite the fact that live fine root biomass constitutes only a small fraction of total stand biomass (McClaugherty et al. 1982; Le Goff and Ottorini 2001).
Black locust is adaptable to environmental extremes such as drought, air pollutants, and high light intensities (Hanover 1989). Various researches have focused on the distribution and dynamics of fine roots of black locust, especially in dry regions (Boring and Swank 1984; Li et al. 2002; Wang et al. 2004; Yan et al. 2008). However, relatively few studies have been carried out about the fine roots of black locust growing in the saline soils. Despite a wealth of information on velvet ash that has been compiled, largely in breeding and cultivation (Copeland 1960; Wang et al. 2007; Yu 1996), no reports are available about the fine roots of velvet ash in salt-affected soils.
The rhizosphere is the zone of soil surrounding a plant root where the biology and chemistry of the soil are significantly influenced by living roots. This zone is about 1–2 mm wide, but has no distinct edge (Bertin et al. 2003). Rather, it is an area of intense biological and chemical activity influenced by compounds exuded by the root, and by microorganisms feeding on the compounds. The chemical components of rhizosphere can be very different from that of bulk soil due to root exudation, nutrient uptake and microorganism activity (Wang and Zabowski 1998). Soil salinity and tree species might affect the physio-chemical characteristics and biological properties of rhizopshere soils of different trees in the same mixed forest. However, less information is available on rhizopshere soil characteristics of black locust and velvet ash in saline soil.
We hypothesized that fine root distribution, characteristics and rhizosphere soil properties differ significantly between black locust and velvet ash in coastal saline soil, which accounts for the differences between the two tree species in growth performance to some extent. To test our hypothesis, we investigated the distribution, morphology, and activity of fine roots as well as rhizosphere soil properties of the two species. The objective was to define the adoptability of two tree species under long-term soil salinity stress in the Yellow River delta.
2 Materials and methods
2.1 Site description
The study was conducted in Gudao township, Dongyin city, Shandong province of north China (37°53´N, 118°48´E, and 2 m above sea level), which is located in the central part of the Yellow River delta. This region has a temperate monsoon climate, with an average annual rainfall of 574.4 mm. The average rainfall is between 364.5 mm for the summer and 20.7 mm for the winter (part of rain falls as snow), respectively. The mean annual temperature is 12.3 °C with January being the coldest month (–3.0 °C) and July the warmest (26.6 °C) and frost occurs only from November through March. The morphology of the region is flat and the soil texture is fluvo-aquic loam. Main characteristics of topsoil (0–20 cm) in the research site included 1.6 g kg–1 organic matter, available nitrogen (N) 75.4 mg kg–1, available phosphorus (P) 4.0 mg kg–1 and available potassium (K) 92.6 mg kg–1 (Lu 1999). The available N, P and K were ammonium and nitrate extracted by potassium chloride, sodium bicarbonate extractable phosphorus (Olsen P) and ammonium acetate extractable potassium, respectively. Soil pH and salt content were 8.8 (1: 2.5 soil/water suspension) and 0.08 mg kg–1, respectively. The mixed forest of black locust and velvet ash was established in 1985 by hand planting one-year-old seedlings in alternate rows. Plant density was 1333 tree ha–1 with 3 m between-row and 2.5 m within-row spacing. After planting of black locust and velvet ash, the mixed forest land were fenced to avoid human disturbance.
2.2 Field investigation and sampling
Field investigation and sampling were designed to compare the differences of black locust and velvet ash in aboveground growth, fine root distribution and rhizosphere soil characteristics. In September 2011, three sampling plots were selected in the mixed stands. Each plot was 20 by 30 m, providing 5 rows of black locust and another 5 rows for velvet ash. For each plot, all trees were measured for height, DBH (diameter at breast height) and canopy area. The numbers of live trees of black locust or velvet ash were recorded to calculate their survival rate. The average height, DBH, and canopy area was 6.53 m, 16.32 cm and 34% for black locust, and 9.05 m, 20.43 cm and 52% for velvet ash, respectively. The survival rates of black locust and velvet ash were 43.3% and 57.4%, respectively.
In each plot, 3 trees of black locust and velvet ash with diameter close to their species average value were randomly selected to sample fine roots and rhizosphere soil. The sampling distance was 0–250 cm from the stem of each selected tree toward the nearest tree of another species. The fine root samples of the tree were collected by excavating the soils from a profile of 50 cm × 60 cm (width × depth) at horizontal distances of 0–50 cm, 50–100 cm, 100–150 cm, 150–200 cm and 200–250 cm from the stem, which were separated into 3 20-cm horizons. Rhizosphere soil was collected according to the procedures described by Wang and Zabowski (1998) in the following way: taking the roots out of the soil with minimum injury, generally shaking the roots until the soil not tightly adhering to roots was removed and then collecting the soil closely adhering to the root system by putting the roots into a paper bag and vigorously shaking them. After washing, fine roots (diameter < 2 mm) in each of 15 sections were manually separated from coarse roots. Color (yellow for velvet roots and grey for black locust roots) was used to distinguish roots of the two species. In each plot, three soil profiles were randomly selected to sample non-rhizosphere soil as control. Soil samples were collected from 0–20 cm, 20–40 cm, 40–60 cm depths with a 5-cm-diameter tube auger after removing all roots from soil to avoid mingling with a small amount of rhizosphere soil adhering to roots.
2.3 Analysis of fine roots and soil sample
Fine roots were separated into live and dead fractions based on the elasticity of their tissues and the color of the cortex (Tufekcioglu 1999). Live roots were yellow (for velvet ash) or grey (for black locust), elastic and free of decay. However, dead roots were brown (for velvet ash) or black (for black locust), broke easily and were in various stages of decay. Only live roots were included in the following analyses. After scanning, they were processed with WinRHIZO (Regents Instruments Inc. Quebec, Canada) to obtain length, surface area, volume and diameter. In order to compare the fine root activity of black locust and velvet ash, a part of fine roots were taken out to determine activity by the TTC (2,3,5-triphenyl tetrazolium chloride) reduction method of Yoshida (1966). The fine-root viability was expressed as the amount of TTC reduced per gram of root fresh mass per hour (µg g–1 h–1). Finally, roots were dried in an oven at 80 °C for 48 hours and weighed. The fine root biomass was expressed as the oven dry weight per soil volume (g l–1). Based on these measures, the three morphological ratios, specific root length (SRL, fine root length to dry weight, m g–1), specific root area (SRA, fine root area to dry weight, cm2 g–1) and specific root volume (SRV, fine root volume to dry weight, cm3 g–1) were calculated.
The properties of rhizosphere soil were analyzed according to standard methods described by Lu (1999). Available N content was determined by an indophenol blue colourimetric method. Available P content was extracted by 0.5 M sodium bicarbonate and measured using ascorbic acid-ammonium molybdate method. To determine available K, rhizosphere soil was extracted by ammonium acetate and determined with a flame spectrophotometer. Total soluble salt content was extracted by water (1:5, w/v) and determined gravimetrically by digesting the soil water extract with H2O2, evaporating to dryness and weighing the residues. Soil pH was measured using an electrode pH-meter in a 1:2.5 soil:water suspension. Soil organic matter was determined using the Walkey-Black method (Walkey and Black 1934).
2.4 Data analysis
The data on root biomass, fine root activity, and soil properties were analyzed as a factorial design with three soil layers and three soil types (control, rhizosphere of velvet ash, and rhizosphere of black locust) as factors. For fine root morphological parameters, an unpaired t test was used to analyze difference for SRL, SRA, SRV and average diameter between black locust and velvet ash. Two-way analysis of variance (ANOVA) was carried out to compare the fine root biomass, fine root activity and rhizosphere soil characteristics of black locust and velvet ash. When the ANOVA analysis found significant differences between treatments, the LSD (Least significant difference) test was conducted to detect differences between individual treatment level means. All statistical analyses were performed at a significance level of p < 0.05. ANOVA and multiple comparisons were performed using SPSS software (version 15.0; SPSS Inc., Chicago, Illinois). All results in figures and tables were given as the mean of three plots. The data of each plot was the mean of 3 trees.
3 Results
3.1 Fine root distribution in soil
Total weights of fine roots in the 0–20 cm soil layer for both black locust and velvet ash were significantly higher than those in the soil layer of 20–40 cm or 40–60 cm, respectively (Fig. 1). Regarding the vertical distribution in the soil profile, fine roots are concentrated into the uppermost soil layer. Compared with black locust, the fine root biomass of velvet ash in the depth of 0–40 cm was significantly higher. However, in the soil layer 40–60 cm the fine root biomass of velvet ash was significantly lower than that of black locust. The average values of fine root biomass were 0.10 g l–1 and 0.12 g l–1 for black locust and velvet ash, respectively. The results indicated that velvet ash had more fine roots than black locust (p < 0.05), mainly distributed in the soil layer of 0–20 cm.
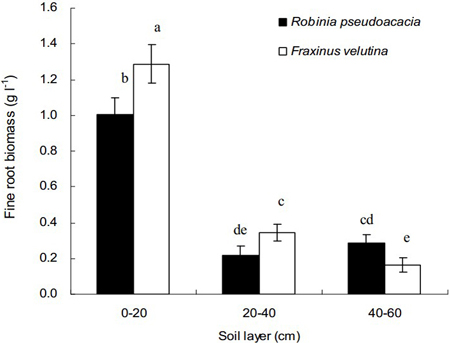
Fig. 1. Vertical distributions of fine root biomass of Robinia pseudoacacia and Fraxinus velutina in the mixed planted forest at different depths of the saline soil. Each vertical bar represents the average of three sampling plots for each tree species. The error bars represent the standard deviation. Bars with different letters are significantly different at p < 0.05 by the least significant difference test.
The distributions of fine roots of black locust and velvet ash in the soil with increasing distance from stem are shown in Fig. 2. The spread distance for velvet ash was 250 cm from stem, much longer than 150 cm for black locust. However, few fine roots were found in the distance of 0–50 cm close to the stem of velvet ash. The highest biomass of fine roots for both tree species was 50–100 cm from the stem. In comparison, velvet ash distributed fine roots within a more sizable zone in the saline soil than black locust.
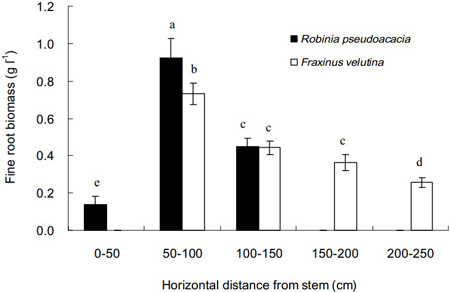
Fig. 2. Horizontal distributions of fine root biomass of Robinia pseudoacacia and Fraxinus velutina in the mixed planted forest at different distances from stem in the saline soil. Each vertical bar represents the average of three sampling plots for each tree species. The error bars represent the standard deviation. Bars with different letters are significantly different at p < 0.05 by the least significant difference test.
3.2 Fine root characteristics
The values of fine root morphological parameters for velvet ash tree (Table 1) showed clearly, that the SRL, SRA and SRV of fine roots were higher than those for black locust. Statistical analysis indicated that the differences in these parameters between the two trees were significant (p < 0.05). However, there was no significant difference in average diameter of fine roots between black locust and velvet ash.
| Table 1. Fine root morphological parameters of black locust and velvet ash. | ||||
| Tree species | Specific root length (m g–1) | Specific root area (cm2 g–1) | Specific root volume (cm3 g–1) | Average diameter (mm) |
| Black locust | 7.08 b a) | 99.92 b | 5.11 b | 0.51 a |
| Velvet ash | 8.91 a | 116.30 a | 7.21 a | 0.55 a |
| a) Different letters in a same column mean significant difference at p < 0.05 level. | ||||
Because the fine roots of black locust and velvet ash were mostly concentrated into the soil profiles within the horizontal distance of 50–100 cm from stem, the fine roots at this distance was selected to compare root activity of the two trees in this study. The decline of root activity with increasing soil depth is observable (Fig. 3). The fine root activity of velvet ash was significantly higher than that of black locust, with an increase of 5.77, 6.95 and 0.63 times in the soil layers of 0–20 cm, 20–40 cm and 40–60 cm depth, respectively. It is suggested that the fine roots of velvet ash have higher viability than black locust in the coastal saline soil.
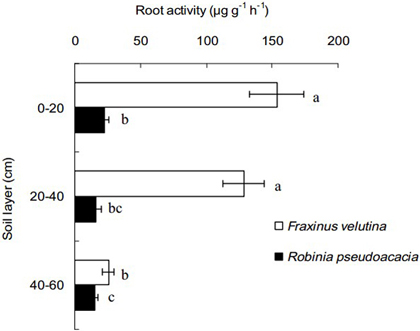
Fig. 3. Fine root activity of Robinia pseudoacacia and Fraxinus velutina in the mixed planted forest within 50–100 cm distance from stem in the saline soil. The value of fine root activity was expressed as the amount of 2,3,5-triphenyl tetrazolium chloride reduced per gram of root fresh mass per hour (µg g–1 h–1). Each horizontal bar represents the average of three sampling plots for each tree species. The error bars represent the standard deviation. Bars with different letters are significantly different at p < 0.05 by the least significant difference test.
3.3 Rhizosphere soil properties
The rhizosphere soil properties of black locust and velvet ash in the mixed forest are presented in Table 2. In all rhizosphere and non-rhizosphere soils, pH and soluble salt content were increased with an increase of soil depth. The contents of organic matter, available N, P and K had the similar pattern of vertical change in the soil profile, i.e. decreasing with increasing soil depth. Compared with the control, rhizosphere soil pH for both species was significantly less in the same soil layer. However, the other five variables of rhizosphere soil for both black locust and velvet ash were higher than those of non-rhizosphere soil.
| Table 2. Soil properties in the mixed planted forest of black locust and velvet ash. | |||||||
| Soil layer (cm) | Soil sample type | pH | Soluble salt (g kg–1) | Organic matter (g kg–1) | Available N (mg kg–1) | Available P (mg kg–1) | Available K (mg kg–1) |
| 0–20 | Control a) | 8.51 a b) | 0.79 c | 16.2 c | 75.39 c | 3.95 b | 92.6 c |
| BRS | 8.27 b | 0.85 b | 21.1 b | 98.34 a | 4.15 b | 113.5 b | |
| VRS | 8.13 c | 0.92 a | 27.5 a | 86.39 b | 5.71 a | 140.3 a | |
| 20–40 | Control | 8.61 a | 0.95 b | 10.8 c | 54.18 c | 3.16 c | 80.6 b |
| BRS | 8.32 b | 0.96 b | 15.8 b | 75.41 a | 3.76 b | 86.4 b | |
| VRS | 8.20 c | 1.34 a | 16.5 a | 64.88 b | 4.74 a | 105.3 a | |
| 40–60 | Control | 8.76 a | 1.63 c | 8.3 c | 36.64 c | 2.67 c | 56.8 b |
| BRS | 8.46 b | 1.76 b | 13.3 b | 55.36 a | 4.15 b | 63.5 a | |
| VRS | 8.22 c | 2.01 a | 14.3 a | 53.28 a | 4.93 a | 66.4 a | |
| a) Control – non-rhizosphere soil, BRS – rhizosphere soil of black locust, VRS – rhizosphere soil of velvet ash. b) Different letters in a same soil layer mean significant difference at p < 0.05 level. | |||||||
In the rhizosphere soil of velvet ash, pH and available N were lower than those of black locust in the same soil depth. Conversely, contents of soluble salt, organic matter, available P and available K were higher in the rhizosphere soil of velvet ash than in black locust soil, respectively. The soil properties in the rhizosphere of both black locust and velvet ash were improved to some extent, due to lower pH and soluble salt content, and higher contents of organic matter, available N, P and K in comparison to non-rhizophere soil. The effects of afforestation on rhizosphere soil properties varied with tree species.
4 Discussion
The pattern of fine root distribution decreasing with soil depth was similar to that in many forest ecosystems (Bennett et al. 2002; Makkonen and Helmisaari 1998). This may reflect the distribution of nutrients returned to the soil by litterfall, canopy leachates and stemflow, and the trophotaxis of fine roots (Yang et al. 2004). Ford and Deans (1997) stated that high concentration of fine roots in the surface soil layer of the forest are related to higher nutrient concentrations when there is enough moisture to encourage decomposition of the organic litter and release of nutrients on the surface soil, particularly during active growth periods. The comparison between black locust and velvet ash showed that a significant difference of fine root biomass existed in the soil profile, which might be mainly ascribed to genetic differences between the tree species (Li et al. 2002). A high tissue mass density is generally associated with species characteristic of stressed environments (Ryster 2006). In costal saline soil of the Yellow River delta, the soluble salt content in the surface soil layer is relatively low compared to the deeper layers (Xing and Zhang 2006). The high concentration of fine roots of both tree species in the surface soil layers of the forest was beneficial to their acclimation to the saline soil. The fine root distribution of both species suggests a strategy of avoiding salinity rather than salt–tolerance. In comparison, velvet ash showed a stronger acclimation response than black locust.
In the horizontal direction, the fine root biomass of black locust was distributed within the distance of 0–150 cm from the tree trunk, mainly within 50–100 cm. The findings are comparable to the results of Wang et al. (2004). They found that root biomass of black locust mainly concentrated within 30–90 cm distance from trees in the southern region of the Loess Plateau, China. Compared with the root biomass in the distance of 50–150 cm, the lower fine root biomass of black locust or velvet ash at short distance (less than 50 cm) from the stem may be due to shading (Kessler 1992) or reduced temperature (Jonsson et al. 1999). However, the lower fine root biomass of black locust or velvet ash at long distance (more than 150 cm) may be due to belowground competition for water and nutrients because of overlap between the two trees. The distribution of fine roots indicated that velvet ash had a more dominant and explorative fine root system than black locust in the coastal saline soil.
Compared with black locust, SRL, SRA and SRV of fine roots were higher for velvet ash. Trees could not only respond to the presence of competitors by changes in their fine root biomass and spatial distribution, but also with modification of fine root morphological traits such as specific root length, root tissue density, and specific area (Kucbel et al. 2002). According to previous researches (Eissenstat and Yanai 1997; Jackson et al. 1997; He 2000), the length and surface area of fine roots are assumed to be proportional to resource acquisition (water and nutrients in soil). Compared with black locust, SRL, SRA and SRV of velvet ash were significantly higher, which indicated that the fine root morphology of velvet ash is beneficial for plant growth. It is well known that root activity is an important factor for growth, vitality and stress tolerance of trees (Lyr and Garbe 2005). In our study, the root activity of velvet ash was much higher than that of black locust in soil profile, indicating that the viability of the former’s fine root was higher than the latter.
Differences in the distribution, morphology, activity of fine root are the response to the differences of the environment, where the fine roots are growing. The planted forests in the Yellow River delta of China are at risk due to high soil salinity and low soil fertility. In the mixed forest selected in this study, the soil type and afforestation time are the same for black locust and velvet ash. The mixed forest was established on native grassland and no fertilizer had been applied to the land. Therefore, the rhizosphere soils of two tree species will have had similar initial conditions, and subsequent differences in soil properties could be mainly ascribed to the effects of tree species. The soil analysis supported our hypotheses that rhizosphere soil properties differed significantly between black locust and velvet ash in the mixed planted forest.
High concentration of fine root biomass in the topsoil can benefit the soil fertility through root exudates, formation of root channel, and return of organic mass and nutrients through root turnover (Yang et al. 2004). In our study, rhizosphere soil of velvet ash at 0–20 cm depth was characterized by higher contents of organic matter, available P and available K than corresponding variables in black locust. Unlike P and K, available N content in rhizosphere soil of velvet ash was lower in contrast with black locust. The possible explanation is that black locust is a tree species with N2-fixing symbionts, which could increase soil N content in rhizosphere. According to the results of Vitousek and Walker (1989), the N2-fixing black locust species can supplement nitrogen pools and increase rates of nitrogen cycling and availability. In this study, some nodules were found on the fine roots of black locust and pink-red pigmentation in the interior was related to the presence of active leghemoglobin, which indicated that the nodules were fixing N2.
Several studies have shown that soil pH can be lower in afforested land in comparison with grassland (Groenendijk et al. 2002; Jobbágy and Jackson 2000). Compared with non-rhizosphere soil, a significant decrease of pH in rhizosphere soil was found in both black locust and velvet ash. The effect in velvet ash was relatively higher. The decrease of soil pH in the rhizosphere was beneficial for plant growth in saline-alkali soils of the Yellow River delta. Unlike soil pH, soluble salt contents in the rhizosphere soil of both trees was higher than the control. The soil salt accumulation in the rhizosphere of some plants has been found by other researchers (Yi et al. 2007; Riley and Barber 1970; Shen and Wang 1993), which possibly is an effective way for plants to increase their tolerance to salt stress. The higher the salt tolerance a halophyte had, the higher the salt content in rhizosphere soil was observed (Zhao and Fan 2007; Sun and Xiao 2004).
The fine root biomass and distribution can be considered as the response to growth competition of tree species. The relatively high fine root biomass of velvet ash compared to black locust, especially concentrating in the 0–20 cm soil layer, could increase its ability to absorb nutrients and water from soil and thus promote aboveground growth, resulting in higher DBH, tree height and canopy area. At the same time, more photosynthate could be allocated into roots. Higher photosynthate availability in the roots may increase C rhizodeposion to soil, which increases soil microbial activity and, consequently, nutrient availability. As a result, the properties of rhizosphere soil were enhanced, in turn, benefiting fine roots. Fine roots always concentrated in the soil layers with the highest nutrient supply (Kucbel et al. 2002).
5 Conclusions
In costal saline soil of the Yellow River delta, the soluble salt content in the surface soil layer is relatively low compared to the deeper layers. In the mixed planted forest, the fine roots of black locust and velvet ash were concentrated in the topsoil of 0–20 cm, which would increase their acclimation capability to the saline soil. The fine root distribution of both species suggests a strategy of avoiding salinity rather than salt tolerance. The fine root distribution and characteristics, and rhizosphere soil properties differed significantly between black locust and velvet ash, which was ascribed to the differences in their current growth status to some extent. By comparison, velvet ash showed stronger acclimation capability to coastal saline soil than black locust, with higher biomass, SRL, SRA, SRV and activity of fine roots as well as longer horizontal spread distance and better properties of rhizosphere soil.
Acknowledgements
This study was funded by the Science and Technology Development Project of Shandong Province, China (2010GNC10942) and supported by CFERN & GENE Award Funds on Ecological Paper. We are grateful to Dr. Austin Himes for English corrections on the manuscript. Finally, we thank the editors and reviewers for their helpful comments on the manuscript.
References
Bennett J.N., Andrew B., Prescott C.E. (2002). Vertical fine root distributions of western redcedar, western hemlock, and salal in old-growth cedar-hemlock forests on northern Vancouver island.Canadian Journal of Forest Research 32: 1208–1216. http://dx.doi.org/10.1139/x02-034.
Bertin C., Yang X.H., Weston L.A. (2003). The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil 256: 67–83. http://dx.doi.org/10.1023/A:1026290508166.
Boring L.R., Swank W.T. (1984). The role of black locust (Robinia pseudoacacia) in forest succession. Journal of Ecology 72: 749–766. http://dx.doi.org/10.2307/2259529.
Copeland H.F. (1960). The reproductive structures of Fraxinus velutina (Oleaceae). Madroño 15(6): 161–72.
Eissenstat D.M., Yanai R.D. (1997). The ecology of root lifespan. Advances in Ecological Research 2: 1–62. http://dx.doi.org/10.1016/S0065-2504(08)60005-7.
Ford E.D., Deans J.D. (1997). Growth of a sitka spruce plantation: spatial distribution and seasonal fluctuations of lengths, weights and carbohydrate concentrations of fine roots. Plant and Soil 47: 463–485. http://dx.doi.org/10.1007/BF00011504.
Francois L.E., Maas E.V. (1999). Crop response and management on salt-affected soils. In: Pessarakli M. (ed.). Handbook of plant and crop stress. Marcel Dekker Press Inc., New York. p. 169–201.
Gebruek T. (2000). Effects of environmental pollution on the genetics of forest trees. In: Young A., Boshier D., Boyle T. (eds.). Forest conservation genetics: principles and practice. CSIRO Publishing, Collingwood. p. 123–133.
Groenendijk F.M., Condron L.M., Rijkse W.C. (2002). Effects of afforestation on organic carbon, nitrogen and sulfur concentrations in New Zealand hill country soils. Geoderma 108: 91–100. http://dx.doi.org/10.1016/S0016-7061(02)00125-8.
Groffman P.M., Gold A.J., Simmons R.C. (1992). Nitrate dynamics in riparian forest: microbial studies. Journal of Environmental Quality 21: 666–671. http://dx.doi.org/10.2134/jeq1992.00472425002100040022x.
Gu J., Liu W., Akinnagbe A., Wang J., Li J., Yang M. (2012). Effect of salt stress on genetic diversity of Robinia pseudoacacia seedlings. African Journal of Biotechnology 11(8): 1838–1847.
Hanover J.W., Mebrahtu T., Bloese P. (1989). Genetic improvement of black locust: a prime agroforestry species. Proceedings of the First Conference on Agroforestry in North America. Guelph, Ontario, Canada. p. 13–16.
He W. (2000). Distribution characteristics of root area of Sabina vulgaris under difference habits. Scientia Silvae Sinicae 36(5): 15–21. [In Chinese].
Jackson R.B., Mooney H.A., Schulze E.D. (1997). A global budget for fine root biomass, surface area, and nutrient contents. Proceedings of the National Academy of Sciences of the United States of America 94: 7362–7366. http://dx.doi.org/10.1073/pnas.94.14.7362 PMid:11038557 PMCid:PMC23826.
Jobbágy E.G., Jackson R.B. (2000). The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10: 423–436. http://dx.doi.org/10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2.
Jonsson K., Ong C.K., Odongos J.C.W. (1999). Influence of scattered néré and karité on microclimate, soil fertility and millet yield in Burkina Faso. Experimental Agriculture 35: 39–53. http://dx.doi.org/10.1017/S0014479799001039.
Kessler J.J. (1992). The influence of karité (Vitellaria paradoxa) and néré (Parkia biglobosa) trees on sorghum production in Burkina Faso. Agroforestry Systems 17: 97–118. http://dx.doi.org/10.1007/BF00053116.
Kucbel S., Jaloviar P., Špišák J. (2011). Quantity, vertical distribution and morphology of fine roots in Norway spruce stands with different stem density. Plant Root 5: 46–55. http://dx.doi.org/10.3117/plantroot.5.46.
Le Goff N., Ottorini J.M. (2001). Root biomass and biomass increment in a beech (Fagus sylvatica L.) stand in North-East France. Annals of Forest Science 58: 1–13. http://dx.doi.org/10.1051/forest:2001104.
Li P., Zhao Z., Li Z.B., Wang N.J. (2002). Advances on the interactional mechanism between root system and ecoenvironment. Journal of Northwest Forestry University 17(2): 26–32. [In Chinese].
Lu R.K. (1999). Analytical methods for soil and agro-chemistry. China Agricultural Science and Technology Publishing House, Beijing, China. 18 p. [In Chinese].
Lyr H., Garbe V. (1995). Influence of root temperature on growth of Pinus sylvestris, Fagus sylvatica, Tilia cordata and Quercus robur. Trees 9: 220–223. http://dx.doi.org/10.1007/BF00195276.
Makkonen K., Helmisaari H.S. (1998). Seasonal and yearly variations of fine root biomass and necromass in a Scots pine (Pinus sylvestris L.) stand. Forest Ecology and Management 102: 283–290. http://dx.doi.org/10.1016/S0378-1127(97)00169-2.
McClaugherty C.A., Aber J.D., Melillo J.M. (1982). The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 63: 1481–1490. http://dx.doi.org/10.2307/1938874.
Mierzwa B., Wdowiak-Wróbel S., Małek W. (2009). Phenotypic, genomic and phylogenetic characteristics of rhizobia isolated from root nodules of Robinia pseudoacacia (black locust) growing in Poland and Japan. Archives of Microbiology 191: 697–710. http://dx.doi.org/10.1007/s00203-009-0500-0 PMid:19669127.
Norby R.J., Jackson R.B. (2000). Root dynamics and global change: seeking an ecosystem perspective. New Phytologist 147: 3–12. http://dx.doi.org/10.1046/j.1469-8137.2000.00676.x.
Riley D., Barber S.A. (1970). Salt accumulation at the soybean (Glycine Max. (L.) Merr.) root-soil interface. Soil Science Society of America Journal 34: 154–155. http://dx.doi.org/10.2136/sssaj1970.03615995003400010042x.
Ryser P. (2006). The mysterious root length. Plant and Soil 286: 1–6. http://dx.doi.org/10.1007/s11104-006-9096-1.
Shen Q.R., Wang J.L. (1993). Distribution characteristics of ions in the rhizosphere of two barley varieties with different salinity tolerance. Acta Pedologica Sinica 30(4): 366–373. [In Chinese].
Singh B. (1975). Role of forestry in mitigating the energy crisis in India. Indian Forester 101: 589–596.
Song Y.M. (2001). Study on salt-tolerance and major planting techniques of Tamarix chinensis. In: Liu M.Y. (ed.). Proceedings of the International Symposium on Halophyte Utilization and Regional Sustainable Development of Agriculture. Meteorology Press, Beijing, China. p. 68–72. [In Chinese].
Sun L., Xiao L. (2004). Studies on the activity and isozyme of superoxide dismutase in chenopodiacea saline species. Journal of Shihezi University (Natural Science) 22(6): 500–503. [In Chinese].
Tufekcioglu A., Raich J.W., Isenhart T.M., Schultz R.C. (1999). Fine root dynamics, coarse root biomass, root distribution, and soil respiration in a multispecies riparian buffer in Central Iowa, USA. Agroforestry Systems 44: 163–174. http://dx.doi.org/10.1023/A:1006221921806.
Vitousek P.M., Walker L.R. (1989). Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen fixation and ecosystem effects. Ecological Monographs 59: 247–265. http://dx.doi.org/10.2307/1942601.
Walkey A., Black I.A. (1934). An examination of Degtjareff method for determing soil organic matter and a proposed modification of the chromic acid titration method. Soil Science 37: 29–37. http://dx.doi.org/10.1097/00010694-193401000-00003.
Wang J.X., Wang D.H., Liu G.Q. (2004). Distribution characteristics of effective root density in man-made forests of Robinia pseudoacacia and Platycladus orientalis. Acta Botanica Boreali-Occidentalia Sinica 24(12): 2208–2214. [In Chinese].
Wang X.P., Zabowski D. (1998). Nutrient composition of Douglas-fir rhizosphere and bulk soil solutions. Plant and Soil 200: 13–20. http://dx.doi.org/10.1023/A:1004240315308.
Wang Y.P., Liu D.X., Sun M.G., Zhou J., Zhang S.P. (2007). Present situation and countermeasure for breeding of Fraxinus velutina. Shandong Forestry Science and Technology 6: 81–83. [In Chinese].
Xing S.J., Zhang J.F. (2006). Land degradation mechanism and ecological rehabilitation techniques in the Yellow River delta. China Forestry Press, Beijing, China. 70 p. [In Chinese].
Yi L.P., Ma J., Li Y. (2007). Soil salt and nutrient concentration in the rhizosphere of desert halophytes. Acta Ecologica Sinica 27(9): 3565–3571. [In Chinese]. http://dx.doi.org/10.1016/S1872-2032(07)60074-2.
Yan H., Su Y.Q, Li J.P., Zhu J.Y., Ji Z.P. (2008). Distribution characteristics of fine root of artificial Robinia pseudoacacia forests and its relation to soil nutrients in the northern slope of Qinling mountains. Research of Soil and Water Conservation 15(3): 65–68. [In Chinese].
Yang Y.S., Chen G.S., Lin P., Xie J.S., Guo J.F. (2004). Fine root distribution, seasonal pattern and production in four plantations compared with a natural forest in subtropical China. Annals of Forest Science 61: 617–627. http://dx.doi.org/10.1051/forest:2004062.
Yoshida T. (1966). Methods of root activity measurements. Journal of the Science of Soil and Manure 37: 63–68. [In Japanese].
Young A. (1988). Agroforestry for soil conservation. Commonwealth Agriculture Bureau, Wallingford, UK. 276 p.
Yu L. (1996). Studies on the cultivation of Fraxinus velutina in saline soils of coastal region. Journal of Liaoning Forestry Science & Technology 4: 13–16. [In Chinese].
Zhang J.F, Li X.F. (2002). Strategies to reclaim and ameliorate saline soil in the Yellow River delta region. In: Chang C., Dobing B. (eds.). International Conference on Environmentally Sustainable Agriculture for Dry Areas for the 3rd Millennium Proceedings. Dobing Enterprises, Canada. p. 264–269.
Zhao K.F., Fan H. (2005). Halophytes and their adaptive physiology to salt habitat. Science Press, Beijing, China. 72 p. [In Chinese].
Total of 47 references
Send to email

