Heartwood, sapwood and bark variation in coppiced Eucalyptus globulus trees in 2nd rotation and comparison with the single-stem 1st rotation
Miranda I., Gominho J., Pereira H. (2015). Heartwood, sapwood and bark variation in coppiced Eucalyptus globulus trees in 2nd rotation and comparison with the single-stem 1st rotation. Silva Fennica vol. 49 no. 1 article id 1141. https://doi.org/10.14214/sf.1141
Highlights
- Coppiced E. globulus trees in the 2nd rotation have similar heartwood and sapwood development as single-stem trees in the 1st rotation
- The initial tree planting density did not influence heartwood development of coppiced E. globulus trees
- Heartwood diameter and height can be modelled with tree diameter and height respectively
- Sapwood width is approximately constant within and between coppice and single-stem E. globulus trees.
Abstract
Coppiced Eucalyptus globulus trees with 18 years in a 2nd rotation were analysed in relation to heartwood, sapwood and bark content taking into account the effect of the initial planting density by using a spacing trial. A total of 25 stumps, with a variable number of stems per stump from 1 to 3, were analysed. Comparison was made to the previous 1st rotation single stem trees, also harvested at 18 years. In the 2nd rotation, the stump density did not significantly affect stem height and diameter, in opposition to the 1st rotation where spacing significantly impacted on tree dimensions. The effect of the initial planting density is somewhat lost in the coppiced stand in relation with i.e. the number of stems per stump. Heartwood was present in all the coppiced trees up to 49.9% of the total tree height and heartwood volume amounted to 38.9–51.7% of the total tree volume. Within the tree, heartwood content decreased from the base upwards, representing, on average, 54.1% at the base and decreasing to 5.1% at 15.3 m. The sapwood width remained relatively constant with an average radial width of approximately 2 cm. The average stem bark content of coppiced trees was 17.4% of the total stem volume. The comparison of heartwood and sapwood development in the coppiced trees did not show significant differences to the 1st rotation trees, nor did the initial spacing. Heartwood diameter could be modelled using the tree diameter both for 1st and 2nd rotation trees.
Keywords
stand density;
stem analysis;
eucalypt silviculture;
harvesting cycle;
within-tree variation
- Miranda, Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Lisboa, 1349-017 Lisboa, Portugal E-mail Imiranda@isa.ulisboa.pt
-
Gominho,
Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Lisboa, 1349-017 Lisboa, Portugal
E-mail
Jgominho@isa.utl.pt

- Pereira, Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Lisboa, 1349-017 Lisboa, Portugal E-mail Hpereira@isa.utl.pt
Received 12 March 2014 Accepted 30 September 2014 Published 21 January 2015
Views 142219
Available at https://doi.org/10.14214/sf.1141 | Download PDF

1 Introduction
Eucalyptus globulus is extensively cultivated for the pulp and paper industry in temperate regions. Portugal was the forerunner in establishing large E. globulus plantations and in setting up an eucalypt-based pulping industry by the second half of the 20th century. Nowadays, in Europe, E. globulus plantations exist mainly in Portugal and Spain, where they cover approximately 1.3 million ha. Most of these eucalypt plantations are managed as a coppice system, with a first cycle of planted single-stem trees followed by two or three coppice regenerated cycles until final harvest, stump removal and replanting.
E. globulus regenerates readily through stump coppice after harvesting (Blake 1983; Little and Gardner 2003). Coppice shoots develop from dormant buds situated in the live bark or from lignotubers i.e. buds found near the junction of root and stem (Reis and Reis 1997). Coppice regrowth benefits from an established root system and biomass production from the first coppice crop usually exceeds that from the single-stem crop of the 1st rotation. Therefore this second rotation without replanting is usually advantageous, provided that the first rotation was well established and stump regrowth was sufficient (Hillis and Brown 1984; El Bassam 2010). Stump mortality also influences the overall production values in the coppice cycles, and the economics of eucalypt forestry has to make the balance between a production increase due to the use of new genetic material with higher productivity and the replanting costs (Guedes et al. 2011). This is especially important in subtropical regions and when development of hybrids and clones is strong, as it is the case of Brazil (Rezende et al. 2005; Gonçalves et al. 2008).
In the usual practice of E. globulus forestry in Europe, the stand density in the coppice cycle is controlled by a selective thinning that is carried out to maintain or increase until about 20% the stand density e.g. by reducing the number of shoots per stump to 2–3 in the first year and 1–2 in the second year (Tomé et al. 2001; Little and Gardner 2003; Soares et al. 2004; Tomé et al. 2007). Similar sprout thinning values are reported in Brazilian and Chilean eucalypt forestry for pulpwood production (Geldres et al. 2004; Souza et al.2012) while a mixed coppice and single stem model for eucalypt stands was also reviewed (Ferraz Filho et al. 2014).
Although considerable work has been done on the wood quality of E. globulus single stem trees (as compiled in Pereira et al. 2010), little is known about the quality of coppiced wood and whether there are differences in relation to the previous single-stem rotation. Information on the influence of silvicultural practices on the technical quality of coppiced trees is also particularly sparse (Gonçalves et al. 2004).
Among the stem properties that impact the quality and value of pulpwood, the amount of heartwood is important because it affects directly the pulp yield and other aspects of pulp and papermaking processes, mainly due to heartwood higher content of extractives (Miranda et al. 2007; Lourenço et al. 2010). Heartwood is present in a significant proportion in the E. globulus stems at the harvest age for pulping in single stem plantations and its proportion was shown to increase with tree size (Gominho and Pereira 2000; Gominho and Pereira 2005; Morais and Pereira 2007). On the contrary, sapwood remains relatively constant as regards its radial width (Gominho and Pereira 2000; Gominho and Pereira 2005; Miranda et al. 2006). There is no research on the development of heartwood and sapwood in coppiced E. globulus.
The objective of the present study is to bring an insight on the stem quality of coppiced E. globulus trees in a 2nd rotation regarding the development of heartwood and sapwood within the tree, as well as of bark, and to analyse the effect of the initial planting density, i.e. stump density, by using a spacing trial for the study. The first single-stem rotation of this trial had been previously studied (Miranda et al. 2009), therefore allowing comparison between the two cutting cycles.
2 Material and methods
2.1 Field sampling
Eucalyptus globulus Labill. trees were harvested at 18 years of age, at the end of the 2nd rotation as a regenerated coppice stand, from a spacing trial located in the site of Alto do Vilão, Furadouro (centre of Portugal, 10 km from the Atlantic Ocean, 39°20´N, 9°15´W, 50 m of altitude). The climate is of the Mediterranean type tempered by oceanic influence, with an annual rainfall of 608 mm and 15.2 °C mean temperature. Predominant soil type is a sandy podzol associated with eutric cambisols.
The Alto do Vilão trial is the oldest eucalypt spacing experiment in Portugal. It was established with commercial E. globulus seeds in March 1975 by the pulping company CELBI (now ALTRI). The trial consists of 2 blocks, each with 5 plots with different tree spacings (mxm): 3×2 (1667 trees ha–1), 3×3 (1111 trees ha–1), 4×3 (833 trees ha–1), 4×4 (625 trees ha–1) and 5×4 (500 trees ha–1). The blocks are located side by side and the spacings distributed according to decreasing density in one of the blocks and the opposite in the other block.
The trial was harvested in March 1993, at 18 years of age, in the 1st rotation, as the planted single-stem stand, and the stumps were left to sprout for a 2nd rotation coppice cycle, as it is usual in the commercial Portuguese eucalypt forestry. The usual practice in the 2nd rotation is to thin the sprouts of each stump by the second year of growth in order to maintain, or slightly increase, the tree density in the plantation.
The thinning of the shoots took place at 2.6 years after harvest, following the usual practice of leaving shoots with dimensions comparable to those of the first rotation of the same age (Soares et al. 2004). This leads to a variable number of shoots per stump, usually ranging from one to three. The end of the 2nd rotation was in 2008, at 18 years of age, when the trial was harvested. Before harvest, the diameter at breast height (d.b.h.) and the total tree height were measured.
Sampling was made in a total of 25 stumps. The stumps sampled were the same of those sampled in the previous 1st cutting, therefore allowing direct comparison between the two rotation cycles, since in both cuttings the trees were 18 year old. As described, the stumps had a variable number of stems, ranging from one to three: in total, 7 stumps with 3 stems/stump, 6 stumps with 2 stems/stump, and 12 stumps with 1 stem/stump. From each sampled tree, 10 cm thick discs were cut at different stem heights: base, 1.30 m and every 2 m along the stem until the top, corresponding to a 7 cm diameter.
2.2 Measurements
The stem discs were analysed using an image-analysis system (Gominho and Pereira 2000). The total cross-sectional area was measured as well as the heartwood, sapwood and bark area. Over-bark and under-bark mean diameters, and bark mean thickness were calculated for all discs using the respective area measurements. The mean bark proportion in the total stem cross-section was calculated. The following variables were calculated for heartwood and sapwood as mean values: heartwood diameter, sapwood radial width, and proportion of heartwood area in the cross-sectional wood area.
The height reached by the heartwood within the tree was estimated as the zero intercept on the linear regression of the heartwood area of the last two stem heights that contained heartwood, and calculated as a percentage of total tree height.
Tree, stemwood and heartwood volumes were calculated using sections corresponding to the different heights of sampling as a cylinder (0–1.3 m), conical sections (1.3–3.3, 3.3–5.3 m, …), and a cone (top) (Gominho and Pereira 2000). Sapwood volume was calculated as the difference of stemwood and heartwood volumes. Bark volume was calculated as the difference of tree and stemwood volumes.
2.3 Comparison with 1st rotation data
The trees harvested in the 2nd cutting had been also measured at the end of the previous 1st cutting, therefore allowing direct comparison between the two cycles since in both cuttings the trees were 18 year old. At harvest of the 1st rotation, the overbark diameter at 1.3 m of height (d.b.h.) and the tree height were determined and the heartwood and sapwood were measured at d.b.h. (Miranda et al. 2009). These values were compared to those obtained for the same stumps in the 2nd rotation.
For comparison of tree and stand volume production between the two cutting cycles, the individual tree total volume was calculated for the 1st and 2nd rotations using the volume equation model developed for E. globulus in Portugal by Tomé et al. (2007), based on d.b.h. and total tree height (h) (V = 0.2105 (d.b.h./100)1.8191 h1.0703).
2.4 Statistical analysis
To estimate the influence of spacing on heartwood formation, one-way ANOVA analysis of variance was done by applying pairwise analysis (Tukey test, p < 0.05). A simple regression model was applied to study the correlation between heartwood volume and tree volume and between heartwood diameter and tree diameter at breast height.
3 Results
3.1 Tree growth and volume
Table 1 summarizes the mean biometric data for the Eucalyptus globulus trees in the 2nd cutting cycle, as coppiced stems from the five stumps per spacing, in relation to b.h. diameter, height and volume (Fig. 1). As referred, the stumps had a variable number of trees, from 1 to 3, and a mean volume per stump was also calculated as the sum of its individual stems. The biometric data for the trees in the 1st rotation are also included in Table 1. The stumps sampled in the 2nd cutting were the same of those sampled in the previous 1st cutting, therefore allowing direct comparison between the two rotation cycles, since in both cuttings the trees were 18 year old.
| Table 1. Biometric data for Eucalyptus globulus trees grown with different planting densities in the 1st cutting cycle, as single stem trees, and in the 2nd cutting cycle, as coppiced trees. The values refer to the same five stumps per spacing in the two cutting cycles. Mean values and standard deviation. | |||||
| 4×5 | 4×4 | 4×3 | 3×3 | 3×2 | |
| 2nd rotation | |||||
| D.b.h. (cm) | 18.5 ± 3.1a | 14.5 ± 2.9a | 18.4 ± 5.0a | 16.8 ± 5.4a | 19.6 ± 3.4a |
| Height (m) | 22.3 ± 2.1a | 20.2 ± 3.0a | 22.3 ± 3.3a | 20.9 ± 5.0a | 24.7 ± 2.9a |
| Tree volume (m3) | 0.30 ± 0.07 | 0.20 ± 0.08 | 0.30 ± 0.15 | 0.25 ± 0.17 | 0.35 ± 0.13 |
| Number of stems per stump | 2.0 ± 1.0 | 2.0 ± 1.0 | 1.6 ± 0.5 | 1.4 ± 0.5 | 1.4 ± 0.5 |
| Volume per stump (m3) | 0.64 ± 0.30 | 0.48 ± 0.39 | 0.55 ± 0.34 | 0.38 ± 0.30 | 0.40 ± 0.16 |
| 1st rotation | |||||
| D.b.h. (cm) | 26.2 ± 5.9 | 22.9 ± 5.5 | 21.8 ± 3.8 | 20.0 ± 5.3 | 17.7 ± 5.4 |
| Height (m) | 26.9 ± 4.1 | 25.7 ± 3.8 | 26.2 ± 2.5 | 24.4 ± 4.5 | 21.2 + 7.4 |
| Tree volume (m3) | 0.67 ± 0.32 | 0.51 ± 0.26 | 0.45 ± 0.19 | 0.38 ± 0.20 | 0.32 ± 0.11 |
| The same letters within rows indicate non-significant differences. | |||||
The average tree height and diameter at breast height for all coppiced trees were 22.9 m and 18.6 cm, respectively, ranging between spacings from 20.2 m to 24.7 m in height and from 14.5 cm to 19.6 cm in breast height diameter. The spacing did not significantly affect the coppice stem height and diameter and it was not a statistically significant source of variation (P = 0.301 and P = 0.540, respectively); for instance, the average tree diameter for the 3×3 and 5×4 spacings was respectively 19.6 cm and 18.5 cm.
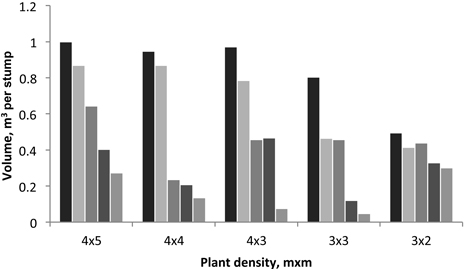
Fig. 1. Variation of tree volume produced by each stump in the 2nd rotation for each spacing. Individual bars represent the five stumps sampled in each spacing plot.
The estimated volume production per ha is shown in Fig. 2 for the 2nd rotation and is compared to that of the 1st rotation. Only in two cases the production was higher in the coppice in relation to the 1st rotation: by 18% and 20% for the 4×3 and 3×2 spacings respectively.
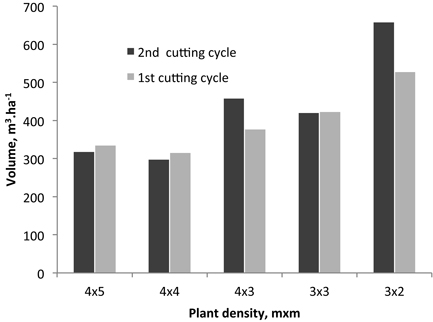
Fig. 2. Volume production per ha for the 2nd and 1st rotations from different initial planting densities.
3.2 Heartwood and sapwood development
Table 2 summarises the mean values of heartwood diameter, proportion and volume, and sapwood radial width at breast height in the 18-year-old coppiced E. globulus trees grown in the different spacing plots. The proportion of heartwood in the cross-section at 1.30 m height level was on average 53% and attained on average 50% of total tree height.
| Table 2. Variation of heartwood diameter, proportion and volume, and sapwood radial width at breast height in the 18-year-old coppiced E. globulus trees grown in the different spacing plots, as well as data published for the 1st rotation (Miranda et al. 2009). Mean of all coppiced trees per spacing and standard deviation. | |||||
| 4×5 | 4×4 | 4×3 | 3×3 | 3×2 | |
| 2nd rotation | |||||
| Heartwood diameter (cm) | 11.5 ± 4.2a | 8.5 ± 3.8a | 10.7 ± 3.0a | 8.4 ± 3.6a | 14.3 ± 1.0a |
| Heartwood proportion (% total area) | 58.8 ± 7.2a | 46.28 ± 12.4a | 51.1 ± 10.4a | 46.4 ± 16.7a | 61.7 ± 6.3a |
| Sapwood width (cm) | 1.7 ± 0.1a | 2.1 ± 0.8a | 1.9 ± 0.6a | 2.0 ± 0.4a | 1.8 ± 0.4a |
| Heartwood height (% total height) | 48.1 ± 6.8 | 42.5 ± 8.6 | 49.3 ± 7.2 | 52.5 ± 8.2 | 57.3 ± 2.4 |
| Heartwood volume (m3) | 0.075 ± 0.04a | 0.051 ± 0.02a | 0.061 ± 0.03a | 0.083 ± 0.06a | 0.097 ± 0.02a |
| Heartwood volume proportion (% total) | 41.2 ± 12.1 | 38.9 ± 11.1 | 42.8 ± 9.7 | 43.5 ± 13.6 | 51.7 ± 5.9 |
| 1st rotation | |||||
| Heartwood diameter (cm) | 15.7 ± 4.2 | 13.3 ± 4.2 | 13.2 ± 3.4 | 11.63 ± 4.21 | 10.31 ± 4.99 |
| Heartwood proportion (% total area) | 62.0 ± 9.9 | 59.4 ± 9.9 | 61.8 ± 8.0 | 57.7 ± 13.9 | 58.4 ± 17.5 |
| Sapwood width (cm) | 2.1 ± 0.7 | 1.9 ± 0.4 | 1.8 ± 0.5 | 1.8 ± 0.4 | 1.5 ± 0.5 |
| The same letters within rows indicate non-significant differences. | |||||
The average stem volume ranged between 0.30 m3 and 0.35 m3 and the proportion of heartwood represented between 38.9% to 51.7% of total tree volume. As shown in Fig. 3, the heartwood volume presents a highly significantly correlation with tree volume (Vheartwood = 0.539 Vstem – 14.291; R2 = 0.862; P < 0.000). The analysis of variance for the heartwood volume showed that stump density was not a significant effect of variation (P = 0.124).
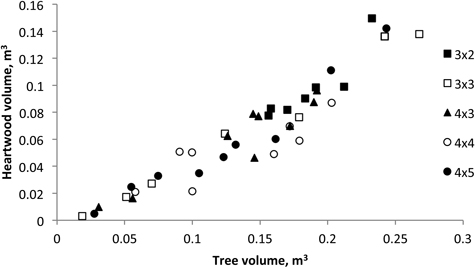
Fig. 3. Heartwood volume versus tree volume of 18-year-old Eucalyptus globulus in the 2nd rotation for the five spacings.
The proportion of heartwood in the stem cross-section decreased steadily from the stem base upwards, approximately following the stem profile (Fig. 4): at the base, heartwood proportion in the cross-section represented on average 54.1% and decreased to 5.1% at 15.3 m.
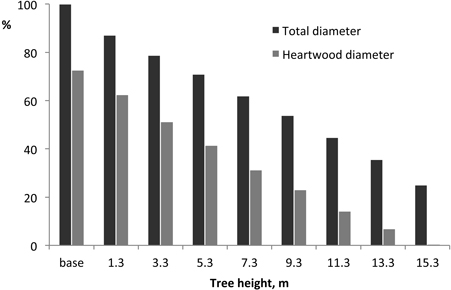
Fig. 4. Variation of tree and heartwood diameter along the tree height. Mean value for all trees, normalised at a reference value of 100 at the base.
There was an influence of stem growth on heartwood development. A high correlation between heartwood diameter and coppiced tree diameter at breast height was found (Fig. 6), corresponding to the following model: Heartwood diameter = –2.622 + 0.875 d.b.h.; R2 = 0.925; P < 0.001 (Fig. 5).
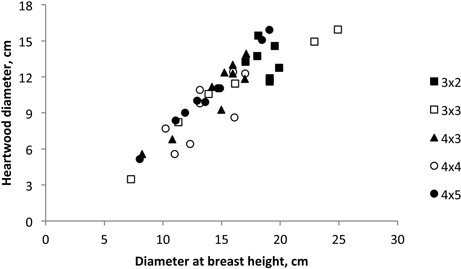
Fig. 5. Heartwood diameter versus tree breast height diameter of 18-year-old Eucalyptus globulus in the 2nd rotation for each stump for the five spacings.
Sapwood width at breast height was in the range of 1.7 to 2.1 cm (Table 2) and no significant relationship was found between sapwood width and tree diameter (R2 = 0.009; P = 0.995). No influence of spacing was found (P = 0.114). The within-tree longitudinal variation of sapwood radial width remained approximately constant along the tree at about 2.0 cm in the lower part of the stem, regardless of tree dimensions, although increasing slightly to 2.6 cm over 10.3 m of tree height (Fig. 7).
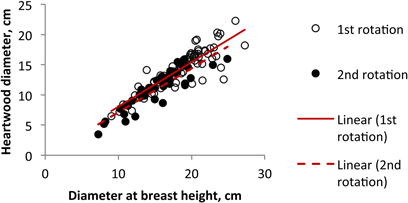
Fig. 6. Relationship between heartwood diameter and tree diameter at b.h. of Eucalyptus globulus at 18 years of age at the end of the 1st and 2nd rotations.
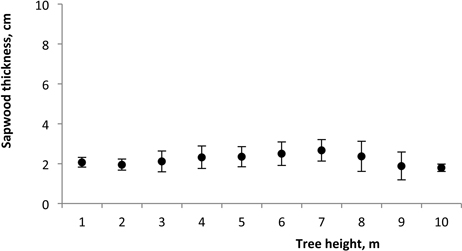
Fig. 7. Variation of sapwood thickness along the tree height of 18-year-old Eucalyptus in the 2nd rotation. Mean value for all trees.
3.3 Bark content and thickness
Within the tree, bark thickness decreased from 12.1 mm at the base to 3.4 mm at the 15.3 m (Fig. 8). The variation of bark thickness with tree height level (X) could be modelled for these 18-year-old coppiced trees by the equation y = 10.987 X–0.584; R2 = 0.914. The average bark volume of 18-year-old E. globulus coppiced trees was 17.4% of the total stem volume while for the 1st rotation at the same age the average bark content was somewhat smaller at 13.8% (Table 3).
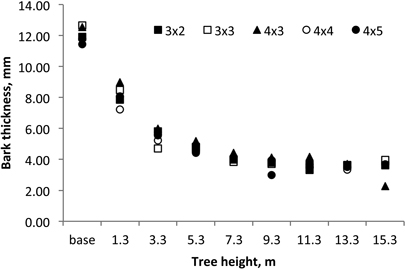
Fig. 8. Axial variation of bark thickness in 18-year-old Eucalyptus globulus in the 2nd rotation for the five spacings.
| Table 3. Variation of bark thickness at b.h. and bark content in % of volume of 18-year-old coppiced (2nd rotation) and single stem (1st rotation) Eucalyptus globulus trees grown in the different spacing plots. Mean of all coppiced trees per spacing and standard deviation. | |||||
| 4×5 | 4×4 | 4×3 | 3×3 | 3×2 | |
| 2nd rotation | |||||
| Bark thickness (mm) | 8.5 ± 2.6a | 7.7 ± 1.5a | 8.6 ± 2.0a | 7.9 ± 2.6a | 8.6 ± 1.6a |
| Bark content (% bolewood) | 17.4 ± 0.1 | 17.4 ± 0.1 | 17.4 ± 0.1 | 17.4 ± 0.2 | 17.4 ± 0.1 |
| 1st rotation | |||||
| Bark thickness (mm) | 10.3 ± 2.5 | 9.2 ± 2.8 | 8.9 ± 2.5 | 7.8 ± 2.6 | 6.6 ± 2.8 |
| Bark content (% bolewood) | 14.4 ± 0.5 | 13.4 ± 2.5 | 14.0 ± 0.7 | 13.8 ± 0.7 | 13.4 ± 1.1 |
| The same letters within rows indicate non-significant differences. | |||||
4 Discussion
In eucalypt plantations managed under a coppice regeneration system, it is usually assumed that the peak production occurs in the second rotation, as a result of a more developed root system that allows a larger supply of mineral nutrients and water, thereby explaining why the initial growth of the coppice shoots is usually more rapid than that of seedlings (Miller and Kauffman 1998). Sims et al. (2001) referred that total biomass yields of the coppice harvest were significantly higher compared to the single stem harvest of 19 Eucalyptus species after 6 years of growth. However stump mortality or destruction by silvicultural operations should be taken into consideration (Camargo et al. 1997; Machado et al. 1990; Stape 1997)
In the present study, the estimated volume production per ha is shown in Fig. 2 for the 2nd rotation (coppice) and is compared to that of the 1st rotation (single stem). An overall increase of production in the coppice was not found and only in two spacing cases the production was higher in relation to the 1st rotation: for the 4×3 and 3×2 spacings the production increased by 18% and 20% respectively. No trend of variation in relation to the original planting density was observed (Fig. 2).
While individual tree growth in diameter and height is influenced by tree competition and therefore by the initial spacing (West 2006), basal area and volume production per unit area will result from the combined effect of number of trees and of their dimensions. The spacing effect in the tree dimensions was clearly seen in the 1st rotation of this trial (Table 1): in the closest 3×2 spacing the trees were smaller and increased regularly with the widening of spacing until the 4×5 spacing (d.b.h. of 17.7 cm and 26.2 cm, respectively).
On the contrary, in the case of the trees sampled at the end of 2nd rotation, the initial planting density, i.e. stump density, did not significantly effect on the coppice stem height and diameter (Table 1). This shows that a coppiced stand somewhat loses the effect of the initial planting density and has a more irregular space occupation by the tree crowns, which will be in relation with the specific local situation i.e. the number of stems per stump. In fact, the tree stand density was changed between the two rotations due to the variable number of sprouts left in each stump (Table 1): for instance, the 1st rotation stand density of 500 trees/ha (4×5 spacing) was increased to 1100 trees/ha in the 2nd rotation. This is also seen by the diversity of tree volume per stump in the different spacings (Fig. 1). The variation in tree density in coppiced regenerated plots was also noticed by António et al. (2007) who reported values between 1605 and 6400 trees per ha in commercial E. globulus plantations in Portugal.
The thinning of sprouts from each stump is therefore an important step to determine production on a unit area base. Since the thinning operation is subjective and at the decision of the forest worker, it may be biased towards an over thinning in wider spacings.
The sampled coppiced trees of E. globulus at the end of the 2nd rotation contained a substantial amount of heartwood: on average 53% of the stem cross-section at breast height level was transformed in heartwood, and representing on average 46% of total tree volume (Table 2). These values are somewhat above those found for E. globulus trees used for pulping at the usual harvest age (9–14 years) where heartwood volume represented 20% to 39% of the stem (Gominho and Pereira 2000; Gominho and Pereira 2005; Morais and Pereira 2007). Miranda et al. (2006) and Miranda et al. (2009) also reported high values of heartwood proportion at breast height, between 58% and 77.5% for 18-year-old E. globulus trees and attaining on average 67.5% of total tree height.
An effect of stump density on the heartwood development was not found in this study: both heartwood volume and diameter at breast height were similar in the five sumps density and the differences were not statistically significant.
The heartwood showed in all cases a similar axial profile within the coppiced trees, with heartwood area decreasing steadily from the stem base upwards and approximately following the stem profile (Fig. 4). Such pattern of heartwood axial variation broadly parallels what has been shown previously for E. globulus (Gominho and Pereira 2000; Miranda et al. 2006; Morais and Pereira 2007) as well as for other eucalypt species e.g. Eucalyptus grandis Hill (Wilkings 1991) and Eucalyptus tereticornis Smith (Purkayastha et al. 1980). This type of within-tree variation of heartwood is generally found in hardwood and softwood species (Hillis 1987) and derive from the process of heartwood formation that starts at a certain tree age and size and progresses outwards in radial direction and upwards in axial direction.
In this study, the heartwood content was found to be positively influenced by the tree growth and a linear relationship between heartwood volume and tree volume was obtained (Fig. 3). The results published by Miranda et al. (2006) for 18-year-old trees at the end of the 1st rotation, also showed a similar linear regression. Similar heartwood dimensional models were also reported for eucalypts with other ages e.g. Gominho and Pereira (2000) reported a positive correlation of heartwood content and growth for 9-year-old E. globulus trees. It was also shown that it was the difference in tree growth that influenced heartwood development, and not the silvicultural treatment e.g. spacing, per se (Gominho and Pereira 2005; Miranda et al. 2006; Morais and Pereira 2012). Thus, in practical terms, heartwood development will be increased through forest practices that increase individual tree growth, such as fertilization and irrigation, or wider spacing, as previously reported (Miranda et al. 2006; Gominho and Pereira 2005).
The comparison of the 2nd rotation in relation to the 1st rotation (Fig. 6) shows that the relation found between heartwood diameter and tree diameter was similar for both rotations, with the increase in heartwood directly related to tree size. Therefore the differences found in the heartwood development between the two rotations cycles should only represent the differences in tree size and do not derive from the type of rotation. Similar results were obtained by António et al. (2007) when developing allometric models for E. globulus, who proposed the use of the same equations for the planted and the coppiced regenerated stands.
The values obtained here for the sapwood width (1.7 to 2.1 cm, Table 2) are in the range of values referred for E. globulus trees at harvest age for pulping in the 1st rotation (Gominho and Pereira 2005; Morais and Pereira 2007), and in the range of 1.5 and 2.1 cm for trees of 18 years of age, also in the end of the 1st rotation (Miranda et al. 2006; Miranda et al. 2009).
The within-tree longitudinal variation of sapwood width remained approximately constant along the tree (Fig. 7). Other studies on sapwood development have also reported an approximately constant width of sapwood along the below-the-crown stem for E. globulus (Gominho and Pereira 2000; Gominho and Pereira 2005; Miranda et al. 2006; Miranda et al. 2007) as well as for Eucalyptus grandis (Bamber 1976; Wilkins 1991). These results indicate that sapwood width in one species may be independent of tree age and dimensions, and support the theory that the amount of sapwood in a tree is related to its conductive needs (Shinozaki et al. 1964). Therefore the formation and development of heartwood progress within the tree to regulate the amount of sapwood (Bamber 1976).
Bark thickness decreased within the tree along the below-the-crown stem (Fig. 8) with a pattern of variation similar for all the coppiced trees and spacings. A similar axial variation in bark thickness was previously reported for E. globulus: 6.5 mm at the base to 1.9 mm at the top (75% of total tree height) in 15-year-old trees (Quilhó et al. 2000; Quilhó and Pereira 2001).
Since tree volume growth is usually estimated by measurements that include the bark (over bark diameter measurements), it is therefore advised to measure bark thickness simultaneously with total tree diameter for obtaining a more accurate estimate of wood volume and avoid over- or under estimates.
The average bark volume of these 18-year-old E. globulus coppiced trees was 17.4% of the total stem volume while for the 1st rotation at the same age the average bark content was somewhat smaller at 13.8% (Table 3).The average bark volume of 18-year-old E. globulus coppiced trees was 17.4% of the total stem volume while for the 1st rotation at the same age the average bark content was somewhat smaller at 13.8% (Table 3). A few studies made on 7 and 11-year-old E. globulus trees indicated mean values of 15.2% of bark content (Ramírez et al. 2009; Miranda et al. 2012). Sansígolo and Ramos (2011) referred values between 13.6% and 15.9% in 4-year-old Eucalyptus grandis, and De Jesus et al. (1988) reported 10–12% for Eucalyptus urophylla Blake at 4 years of age. In all cases, the proportion of bark in eucalypt trees is substantial and allows considering a valorization of this biomass residue, e.g. by integration into a biorefinery concept (Miranda et al. 2013).
5 Conclusions
The coppiced E. globulus trees in a 2nd rotation showed a similar heartwood and sapwood development as the single-stem trees in the first rotation. The initial tree planting density did not influence heartwood development in the coppice. Heartwood dimensions within the tree were determined by tree size, and heartwood diameter could be modelled with tree diameter and heartwood height with tree height. The sapwood width was approximately constant within and between trees, and in both cutting cycles, therefore showing its species’ specificity.
Coppice E. globulus trees are similar to single stem trees as regards tree development and heartwood formation. The silvicultural operation of sprout thinning at the beginning of the 2nd rotation influenced the stand tree density and therefore the wood production will depend on the thinning decisions i.e. number of sprouts left on the stump.
References
António N., Tomé M., Tomé J., Soares P., Fontes L. (2007). Effect of tree, stand, and site variables on the allometry of Eucalyptus globulus tree biomass. Canadian Journal of Forest Research 37(5): 895–906. http://dx.doi.org/10.1139/X06-276.
Bamber R.K. (1976). Heartwood, its function and formation. Wood Science and Technology 10: 1–8.
Blake T.J. (1983). Coppice systems for short-rotation intensive forestry: the influence of cultural, seasonal and plant factors. Australian Forest Research 13(3/4): 279–291.
Camargo F.R.A., Silva C.R., Stape J.L. (1997). Resultados experimentais da fase de emissão de brotação em Eucalyptus manejado por talhadia. Série Técnica IPEF 11(30): 115–122.
De Jesus R.M., Rossmann N.C., Brouard J.S. (1988). Eucalyptus/Leucaena mixture experiment – wood properties. IPEF, Piracicaba 39: 49–52.
El Bassam N. (2010). Handbook of bioenergy crops. A complete reference to species, development and applications. Earthscan, London and Washington DC. 516 p.
Ferraz-Filho A.C., Scolforo J.R.S., Mola-Yudego B. (2014). The coppice-with-standards silvicultural system as applied to Eucalyptus plantations – a review. Journal of Forestry Research 25(2): 237−248. http://dx.doi.org/10.1007/s11676-014-0455-0.
Geldres E., Schlatter J.E., Marcoleta A. (2004). Coppice options for three Eucalyptus species, a case in the Osorno Province, X Region. Bosque 25(3): 57–62. http://dx.doi.org/10.4067/S0717-92002004000300006.
Gominho J., Pereira H. (2000). Variability of heartwood content in plantation grown Eucalyptus globulus Labill. Wood and Fiber Science 32(2): 189–195.
Gominho J., Pereira H. (2005). The influence of tree spacing in heartwood content in Eucalyptus globulus Labill.. Wood and Fiber Science 37(4): 582–590.
Gonçalves J.L.M., Stape J.L., Laclau J-P., Smethurst P., Gava J.L. (2004). Silvicultural effects on the productivity and wood quality of eucalypt plantations. Forest Ecology and Management 193(1/2): 45–61. http://dx.doi.org/10.1016/j.foreco.2004.01.022.
Gonçalves J.L.M., Stape J.L., Laclau J.P., Bouillet J.P., Ranger J. (2008). Assessing the effects of early silvicultural management on long-term site productivity of fast-growing eucalypt plantations: the Brazilian experience. Southern Forests 70(2): 105–118. http://dx.doi.org/10.2989/SOUTH.FOR.2008.70.2.6.534.
Guedes I.C.L., Coelho Júnior L.M., Oliveira A.D., Mello J.M., Rezende J.L.P., Silva C.P.C. (2011). Economic analysis of replacement regeneration and coppice regeneration Eucalyptus stands under risk conditions. Cerne, Lavras 17(3): 393–401.
Hillis W.E. (1987). Heartwood and tree exudates. Springer-Verlag, Berlin. 268 p.
Hillis W.E., Brown A.G. (1984). Eucalypts for wood production. CSIRO/Academic Press, Melbourne. 434 p.
Little K.M., Gardner R.A.W. (2003). Coppicing ability of 20 Eucalyptus species grown at two high-altitude sites in South Africa. Canadian Journal of Forest Research 33(2): 181–189. http://dx.doi.org/10.1139/x02-170.
Lourenço A., Gominho J., Pereira H. (2010). Pulping and delignification of sapwood and heartwood from Eucalyptus globulus. Journal of Pulp and Paper Science 36(3/4): 63–69.
Machado C.C., Ignácio A.S., Vale A.B., Souza Júnior H.S.S. (1990). Efeito da extração de madeira com guincho arrastador na brotação do Eucalyptus alba. Revista Árvore 14(1): 55–60.
Miller P.M., Kauffman J.B. (1998). Seedling and sprout response to slash-and-burn agriculture in a tropical deciduous forest. Biotropica 30(4): 538–546. http://dx.doi.org/10.1111/j.1744-7429.1998.tb00094.x.
Miranda I., Gominho J., Lourenço A., Pereira H. (2006). The influence of irrigation and fertilization on heartwood and sapwood content in 18 year old Eucalyptus globulus Labill. trees. Canadian Journal of Forest Research 36(10): 2675–2683. http://dx.doi.org/10.1139/x06-130.
Miranda I., Gominho J., Lourenço A., Pereira H. (2007). Heartwood, extractives and pulp yield of three Eucalyptus globulus clones grown in two sites. Appita Journal 60(6): 485–500.
Miranda I., Gominho J., Lourenço A., Pereira H. (2009). Variation of heartwood and sapwood in 18-year-old Eucalyptus globulus trees grown with different spacings. Trees – Structure and Function 23: 367–372. http://dx.doi.org/10.1007/s00468-008-0285-9.
Miranda I., Gominho J., Pereira H. (2012). Incorporation of bark and tops in Eucalyptus globulus wood pulping. Bioresources 7(3): 4350–4361.
Miranda I., Gominho J., Mirra I., Pereira H. (2013). Fractioning and chemical characterization of barks of Betula pendula and Eucalyptus globulus. Industrial Crops and Products 4: 299–305. http://dx.doi.org/10.1016/j.indcrop.2012.04.024.
Morais C.M., Pereira H. (2007). Heartwood and sapwood variation in Eucalyptus globulus Labill. trees at the end of rotation for pulpwood production. Annals of Forest Science 64: 665–671. http://dx.doi.org/10.1051/forest:2007045.
Morais M.C., Pereira H. (2012). Variation of extractives content in heartwood and sapwood of Eucalyptus globulus trees. Wood Science and Technology 46(4): 709–719. http://dx.doi.org/10.1007/s00226-011-0438-7.
Pereira H., Miranda I., Gominho J., Tavares F., Quilhó T., Graça J., Rodrigues J., Shatalov A., Knapic S. (2010). Qualidade tecnológica do eucalipto Eucalyptus globulus. Centro de Estudos Florestais, ISA UTL, Lisboa. 377 p.
Purkayastha S.K., Agrawal S.P., Tandon R.D., Chauham L. (1980). Variation in the proportion of heartwood in Eucalyptus tereticornis. Indian Forester 106(7): 466–473.
Quilhó T., Pereira H. (2001). Within and between-tree variation of bark content and wood density of Eucalyptus globulus in commercial plantations. IAWA Journal 22(3): 255–265.
Quilhó T., Pereira H., Richter H. (2000). Within-tree variation in phloem cell dimensions and proportions in Eucalyptus globulus Labill. IAWA Journal 21(1): 31–40.
Ramírez M., Rodriguez J., Balocchi C., Peredo M., Elissetche J.P., Mendonça R., Valenzuela S. (2009). Chemical composition and wood anatomy of Eucalyptus globulus clones: variations and relationships with pulpability and handsheet properties. Journal of Wood Chemistry and Technology 29(1): 43–58. http://dx.doi.org/10.1080/02773810802607559.
Reis G.G., Reis M.G.F. (1997). Fisiologia da brotação de eucalipto com enfase nas suas relações hídricas. Série Técnica IPEF 11(30): 9–22.
Rezende J.L.P., Souza A.N., Oliveira A.D. (2005). The optimal time for substitution of Eucalyptus spp. plantations – the technological progress case. Cerne, Lavras 11(1): 1–15.
Sansígolo C.A., Ramos E.S. (2011). Quality of wood and pulp from a clone of Eucalyptus grandis planted at three locations. Cerne, Lavras 17: 47–60.
Shinozaki K., Yoda K., Hozzumi K., Kira T. (1964). A quantitative analysis of plant form – pipe model theory. I. Basic analysis. Japanese Journal of Ecology 14(3): 97–105.
Sims R.E.H., Maiava T.G., Bullock B.T. (2001). Short rotation coppice tree species selection for woody biomass production in New Zealand. Biomass and Bioenergy 20(5): 329–335. http://dx.doi.org/10.1016/S0961-9534(00)00093-3.
Soares P., Aires N., Tomé M., Araújo C., Pina J.P. (2004). Analysis of the two first cutting cycles of an Eucalyptus globulus spacing trial. In: Borralho N.M.G., Pereira J.S., Marques C., Coutinho J., Madeira M., Tomé M. (eds) IUFRO Conference. Eucalyptus in a Changing World. RAIZ, Instituto Investigação da Floresta e Papel, Portugal. p. 283–289.
Souza F.C., Reis G.G., Reis M.G.F., Leite H.G., Alves F.F., Faria R.S., Pereira M.M. (2012). Survival, sprout number and diameter growth of coppice and intact plants of eucalypt clones. Floresta e Ambiente 19(1): 44–54. http://dx.doi.org/10.4322/floram.2012.006.
Stape J.L. (1997). Planejamento global e normatização de procedimentos operacionais da talhadia simples em Eucalyptus. Série Técnica IPEF 11(30): 51–62.
Tomé M., Ribeiro F., Soares P. (2001). O modelo GLOBULUS 2.1. Relatórios Técnico – Científicos do GIMREF, nº1/2001. Dep. Eng. Florestal, ISA, Lisboa. 189 p.
Tomé M., Tomé J., Ribeiro F., Faias S. (2007). Equação de volume total, volume percentual e de perfil do tronco para Eucalyptus globulus Labill. em Portugal. Silva Lusitana 15(1): 25–39.
West P.W. (2006) Growing plantation forests. Springer-Verlag, Berlin–Heidelberg–New York. 304 p.
Wilkins A.P. (1991) Sapwood, heartwood and bark thickness of silvicultural treated Eucalyptus grandis. Wood Science and Technology 25(6): 415–423. http://dx.doi.org/10.1007/BF00225234.
Total of 43 references

