Gap closure process by lateral extension growth of canopy trees and its effect on woody species regeneration in a temperate secondary forest, Northeast China
Lu D., Zhu J., Sun Y., Hu L., Zhang G. (2015). Gap closure process by lateral extension growth of canopy trees and its effect on woody species regeneration in a temperate secondary forest, Northeast China. Silva Fennica vol. 49 no. 5 article id 1310. https://doi.org/10.14214/sf.1310
Highlights
- Gap closure process by lateral extension growth can be described by quadratic functions
- Large gaps (514–621 m2) had higher closure rates but lower closure percentages compared with middle (174–321 m2) and small gaps (68–125 m2)
- Gaps promoted woody species regeneration in early stage
- Large and middle gaps would provide opportunities for filling regeneration, but regeneration in small gaps may eventually fail.
Abstract
Gap formation and its effects on regeneration have been reported as being important in forest development, but seldom studies concentrated on the gap closure process by lateral extension growth of canopy trees surrounding gaps. We monitored the closure process of 12 artificial gaps for 7 years with three size classes: small (from 68 m2 to 125 m2), middle (from 174 m2 to 321 m2), and large (from 514 m2 to 621 m2); and investigated the regeneration twice in a temperate secondary forest, Northeast China. The closure process can be described through quadratic functions, which showed the closure rates slowed down with gap ages. Large gaps had a higher closure rate (39 m2 a–1) than middle gaps (25 m2 a–1) and small gaps (11 m2 a–1). According to the quadratic equations, the lateral growth could last 11, 13 and 16 years for small, middle and large gaps with a remaining size of 12, 69 and 223 m2, respectively. As expected, regeneration exhibited the highest seedling density and volume in large gaps. There was no significant difference in regeneration density between middle gaps, small gaps and forest understory in the final investigation; but the volume of regenerated woody species increased significantly from small gaps to large gaps compared with forest understory. These results may provide references on the choice of appropriate gap sizes to promote the regeneration in temperate secondary forests.
Keywords
canopy closure;
recruitment;
canopy opening;
opening size;
crown expansion;
duration;
hemispherical photograph
- Lu, State Key Laboratory of Forest and Soil Ecology, Qingyuan Forest CERN, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China; University of Chinese Academy of Sciences, Beijing 100049, China E-mail delianglu14@hotmail.com
-
Zhu,
State Key Laboratory of Forest and Soil Ecology, Qingyuan Forest CERN, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China
E-mail
jiaojunzhu@iae.ac.cn

- Sun, State Key Laboratory of Forest and Soil Ecology, Qingyuan Forest CERN, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China E-mail yirongsun@iae.ac.cn
- Hu, Chinese Research Academy of Environmental Sciences, Beijing 100012, China E-mail lilehu@gmail.com
- Zhang, State Key Laboratory of Forest and Soil Ecology, Qingyuan Forest CERN, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China; University of Chinese Academy of Sciences, Beijing 100049, China E-mail zgq04713@163.com
Received 5 February 2015 Accepted 20 August 2015 Published 27 October 2015
Views 185694
Available at https://doi.org/10.14214/sf.1310 | Download PDF

1 Introduction
Canopy gaps are the predominant and almost permanent small-scale disturbances in many forest ecosystems (Runkle 1982; Denslow 1987; Brokaw and Busing 2000). The formation of gaps has been widely recognized as a crucial process in driving “the forest cycle” (Whitmore 1989), which is defined as a cycle initiated by disturbance in forests, and can be roughly divided into three phases: gap, building, and mature phases (Watt 1947; Whitmore 1989). Compared with some large scale and episodic disturbance events such as fire, hurricane and flood, canopy gaps may be more likely to maintain the diversity and stability of forest ecosystems (Schumann et al. 2003; Gutiérrez et al. 2008). Thus, gap disturbance has been an important silvicultural strategy to sustain natural forest structure (Long 2009) and facilitate forest restoration (Hartshorn 1989) in forest management practices recent years (Schliemann and Bockheim 2011; Wang and Liu 2011; Koivula et al. 2014). Gap formation and closure processes characterize the gap dynamics (Yamamoto 2000), and are important to forest development and succession (Weber et al. 2014). Gap formation changes the forest structure and micro-environmental conditions, resulting in increasing light intensity, soil moisture and nutrients, and additional growing space (Dupuy and Chazdon 2008; Zhang et al. 2013; Vilhar et al. 2014), which promote the regeneration of plants within the gaps (Zhu et al. 2003; Amir 2012). However, gap closure process largely decides how long this mosaic of regeneration phase may last (Brokaw 1985), and whether the regeneration succeeds or not (Runkle and Yetter 1987).
Many studies focused on gap regeneration took little consideration about the gap closure process. Researchers tried to find out the optimal canopy size for successful regeneration (Arevalo and Fernandez-Palacios 2007; Kern et al. 2013). However, more different or even contradictory results increase the uncertainty of gap regeneration (Zhu et al. 2014a). For example, one study claimed that only shade-intolerant species could benefit from large gaps (Chazdon 1986). But other research reported that some shade-tolerant species also grew better in large gaps than in the forest understory (Kern et al. 2012). Moreover, most of the publications confirmed the generally positive effects of gaps on plant regeneration (Denslow 1987; Zhu et al. 2014a). Yet, negative effects on plant regeneration have also been reported (Arevalo and Fernandez-Palacios 2007). Although regeneration within gaps is important to maintain species diversity and promote forest restoration (Schnitzer and Carson 2001; Zhu et al. 2003; Hökkä and Mäkelä 2014), the lateral extension process during the gap duration is also of great importance. Lateral extension growth of canopy trees surrounding gaps influences the light regimes of regeneration layer within gaps (Valverde and Silvertown 1997). Moreover, whether a seedling or sapling colonizes the gap or not depends on not only its height growth rate, but also the lateral extension rate of the trees surrounding gaps (Ogden et al. 1991). Rates of gap closure are important to the understanding of regeneration dynamics within gaps (van der Meer and Bongers 1996). Neglecting lateral growth therefore might overlook the real effects of forest gaps on plant regeneration in both spatial and temporal scales (Rentch et al. 2003).
It is generally recognized that small gaps would close by lateral extension growth of canopy trees surrounding gaps, and large gaps would close finally by filling regeneration of seedlings or saplings within gaps (Runkle 1982). However, rates of lateral extension growth differ in different climate zones and forest types. For example, gaps in boreal coniferous forests exist longer than in temperate or tropical forests due to slower lateral growth rates (Bartemucci et al. 2002). In a tropical rain forest of French Guiana, canopy gaps with a radius of 3 m closed through lateral extension after 5–6 years (van der Meer and Bongers 1996). In a sub-alpine forest in New Zealand, canopy gaps with a radius of 2 m would need 31 years to close, and a large canopy gap with a radius of 7 m would take more than 100 years to close given the same lateral growth rate (Ogden et al. 1991). Furthermore, there is also considerable variability of lateral growth rates among gaps with different sizes and ages in the same study area (Runkle and Yetter 1987; Rentch et al. 2003). Thus, detailed quantification of gap closure process by lateral extension growth under similar conditions is needed.
The observation results of repeated disturbances in a tropical forest suggested that the trees surrounding large gaps were prone to fall into the pre-existing gaps (Young and Hubbell 1991). This adds the uncertainty of gap duration. Gap dynamics in a landscape level have been detected widely recent years with the help of remote sensing technology (Andersen et al. 2014). For example, Vepakomma et al. (2012) characterized the gap dynamics over a 9-year period in a natural old-growth boreal forest based on Lidar monitoring, and found that gaps changed in various ways. However, these large-scale studies may not be suitable for acquiring detailed closure information which is closely related to species regeneration (Getzin et al. 2014). Moreover, long monitor interval (usually >5 years) may obscure or miss the closure process of small gaps.
Therefore, long-term successional monitoring of gap closure process is essential for detecting canopy gap dynamics (Kathke and Bruelheide 2010) and its relationship with regeneration (Weber et al. 2014). However, few studies are available about the detailed gap duration and lateral closure process (Valverde and Silvertown 1997; Webster and Lorimer 2005), especially in temperate secondary forests, which originated from natural regeneration of virgin forests after destructive disturbances of human beings (Zhu et al. 2007). Case studies are necessary to provide a more detailed picture of gap changes with time. In this study, we monitored the closure process of canopy gaps with different sizes and investigated the regeneration within gaps in a secondary forest. Our aims are to (1) quantify lateral extension growth of canopy trees surrounding gaps, and (2) detect regeneration dynamics of woody species within gaps with gap closure. We hypothesize that (1) the rates of lateral extension growth in large gaps are higher than small gaps, but small gaps need less time to close relative to large gaps due to smaller sizes; and (2) gap formation significantly promotes woody species regeneration, especially in large gaps, however, the effects of gaps may gradually weaken and eventually disappear with the development of gap. This work can further help to understand the effects of gap closure on regeneration and may provide important insights into forest management.
2 Material and methods
2.1 Study area
The study was performed at Qingyuan Forest CERN, Chinese Academy of Sciences (CAS), in a mountainous region of Liaoning Province, Northeast China (41°51´N, 124°54´E, 500–1100 m a.s.l.). The climate is continental monsoon with a strong windy spring, a warm and humid summer, and a dry and cold winter. Mean annual temperature is 4.7 °C. The historic minimum temperature was −37.6 °C (January) and maximum temperature was 36.5 °C (July). Mean annual precipitation is 811 mm, 80% of which falls between June and August. The frost-free period is about 130 days (Zhu et al. 2014b). The predominant soil is a clay loamy soil (sand: 25.6%, silt: 51.2%, clay: 23.2%) (Yan et al. 2012). Most of the forests at Qingyuan Forest CERN, CAS grew after a complete fire disturbance in 1950s, forming a typical secondary forest ecosystem. The forests are mainly composed of mixed broadleaved tree species, and dominated by ash (Fraxinus rhynchophylla Hance), maple (Acer mono Maxim.), and Mongolian oak (Quercus mongolica Fisch.). Ash and Mongolian oak are shade-intolerant species, and maple is shade-tolerant species. These woody species are widespread in Northeast China, Russia, Japan, Mongolia, and Korean Peninsula.
2.2 Gap creation
Gaps were created in December (winter) of 2004. Twelve canopy gaps were randomly assigned to 12 locations whose site conditions such as slope, aspect, altitude, and tree species composition were similar to each other (Table 1). Among these 12 gaps, we set three size classes (i.e. 3 treatments): two large gaps (L, i.e. 2 replications, the same below), four middle gaps (M), and six small gaps (S), with ratios of gap diameters to mean height of dominant trees surrounding the gap of 1.5, 1.0, and 0.5, respectively. Each gap was at least 20 meters apart (more than the dominant tree height). We assumed that only the gap size not the shape or other gap characteristics changed between treatments (York et al. 2004) because we tried our best to create the gaps with similar shapes. We also set three control plots (24 m × 24 m) in the forest understory.
| Table 1. General description of the twelve artificial gaps created in December 2004 at Qingyuan Forest CERN, CAS. | ||||||
| Canopy gap | Area (m2) | Mean height of canopy trees surrounding gaps (m) | Slope (o) | Aspect (o) | Elevation (m) | |
| L1 | 513.9 | 19 | 17 | 170 | 650 | |
| L2 | 621.1 | 17 | 23 | 150 | 670 | |
| M1 | 267.3 | 17 | 24 | 140 | 640 | |
| M2 | 174.1 | 16 | 20 | 155 | 690 | |
| M3 | 307.9 | 16 | 25 | 145 | 673 | |
| M4 | 321.2 | 17 | 25 | 160 | 681 | |
| S1 | 83.9 | 16 | 20 | 170 | 630 | |
| S2 | 75.5 | 17 | 22 | 140 | 640 | |
| S3 | 68.4 | 16 | 23 | 170 | 675 | |
| S4 | 86.4 | 18 | 20 | 150 | 634 | |
| S5 | 113.8 | 17 | 26 | 145 | 655 | |
| S6 | 124.5 | 16 | 24 | 165 | 669 | |
| L1 – L2 = large gaps M1 – M4 = middle gaps S1 – S6 = small gaps | ||||||
All target trees for creating gaps were cut using a chainsaw, which may be more appropriate to reduce the soil destruction. In addition, it was winter when we created the forest gaps (the soil was frozen). Therefore, the negative effects of tree removing on the environmental conditions within the gaps were minimized. All the felled trees were removed out of the gaps manually.
2.3 Gap monitoring
Hemispherical photographs were taken at each gap center (Fig. 1a) and control plot center (forest understory) (Fig. 1b) 1.0 m above the ground by using a Nikon Coolpix 995 fitted with an FC-E8 fisheye lens. The lens was set to a small aperture and focused on infinity (Frazer et al. 2001; Hu et al. 2009). The tops of photographs were oriented towards magnetic north (Hu et al. 2009). Images were recorded in 2048 × 1536 pixels, the highest resolution setting possible on the camera. Maximum resolution was used because image analysis is performed based on pixel-by-pixel methods, and image resolution is a major factor reflecting the quality of hemispherical photographs (Jelaska et al. 2006; Brusa and Bunker 2014). We took the hemispherical photographs on uniformly overcast days between July and August from 2005 to 2011. All hemispherical photographs were taken at the same center points, which were permanently marked for exactly relocation.
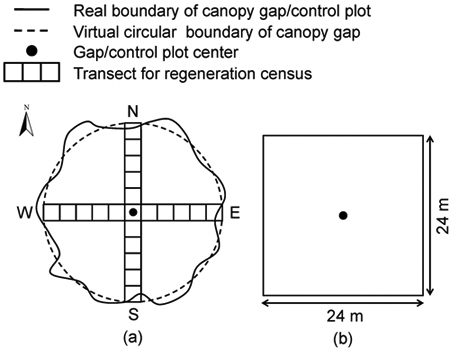
Fig. 1. The schematic of the experimental design: (a) canopy gap and (b) control plot.
The monitoring was continued until the end of the 7th growing season (2011), when the regenerated saplings in large gaps shaded the fisheye lens and prevented us from taking hemispherical photographs. We tried to bend these trees to ensure the edge of canopy gaps clearly and completely shown in the viewfinder, but failed. Finally, a total of 105 effective hemispherical photographs were selected (84 images in gaps and 21 images in the understory) for the analysis in this paper. All these photographs were the clearest ones at each plot during the monitoring years.
Census of regenerated seedlings and saplings in gaps was conducted at the end of the second (2006) and the seventh (2011) growing season. Strips of 2-m width through the gap center in east–west and south–north transects were designed for the census (Fig. 1a). Gap positions were delineated by dividing at drop line in the east–west and south–north transects into continuous sub-plots in interval of 2 m. The tree species, collar diameter and height of all regenerated woody species were measured. The height of each seedling and sapling was determined by measuring the distance from the forest floor (soil surface) to the shoot tip, or the top part. The volume (per square meter) of regenerated seedling and sapling was calculated by multiplying the basal area of individual and its height. These data were used for characterizing regeneration dynamics of woody species within different gaps.
2.4 Photograph analysis
Hemispherical photographs were analyzed in two ways.
First, hemispherical photograph method (HPM) (Hu et al. 2009) was used to calculate canopy gap size. We positioned one point of each border tree’s crown (Pc) where the distance is nearest to the center of the images, and recorded its coordinate (in pixels) by using Adobe Photoshop CS2 software. However, it is sometimes difficult to distinguish the border trees. When this happened, we selected more possible points rather than missed any potential border tree. Based on these coordinates, the radial distance (r) of the projected point to the image center was measured, and then the zenith angle (θ) was approximated by using the following formula:
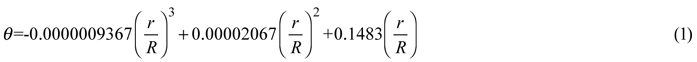
where R is the radial distance in the image when θ equals 90.
The horizontal distance (Dɑ) from the actual Pc to the camera location was obtained by:

where H and h are the height of Pc and camera above the ground; α is the azimuth, β is the aspect of the slope and γ is the gradient of the slope.
Finally, we estimated the size (A) of the canopy opening as:

where n is the number of border trees and the first point (i = 1) is also the last point (i = n + 1), ɑ(i) and Dɑ(i) are the azimuth and horizontal distance of Pc(i).
Detailed calculating processes refer to Hu et al. (2009).
Based on these results, gap closure rate (c) (m2 a–1) was acquired:

where s1 represents the canopy gap size in a previous year, s2 in a subsequent year, and t is the time interval between the observed s1 and s2.
Second, we calculated canopy openness, a proxy for light availability (Kobe and Hogarth 2007), by using Gap Light Analyzer v.2.0 (Frazer et al. 1999). For minimizing and systematizing errors on selecting thresholds, all image-analysis process was conducted by the same person (Forrester et al. 2014).
It is difficult to define a precise gap closure time, because no universal lower limit of gap size is available now. We evaluated gap closure by comparing the canopy openness between gap center and forest understory because the gap sizes were positively correlated with canopy openness (van der Meer and Bongers 1996) and the canopy openness in gap centers diminished gradually and finally reached values similar to the forest understory (van der Meer and Bongers 1996).
2.5 Statistical analysis
T-test was used to analyze canopy openness variation between canopy gaps and forest understory. When an insignificant t-value (p > 0.05) was acquired, we recognized gaps had reached a closed phase. Simple linear regression was used to determine the relationship between gap size and canopy openness. Quadratic function was used to describe gap size variation of different size classes. One-way ANOVA and the LSD test were used to distinguish woody species regeneration (density, volume) among gaps of different size classes and forest understory at two points of time (2006 and 2011). Difference at a level of p < 0.05 was considered significant. All statistical tests were carried out in SPSS 16.0 (SPSS Inc., Chicago, USA).
3 Results
3.1 Gap closure process of lateral extension growth
Gap size decreased with the gap age (Fig. 2). The decreasing rate can be expressed approximately as time-dependent quadratic function curves (Table 2) for small, middle, and large gaps after gap formation. The mean closure rate of large gaps (39 m2 a–1) was significantly higher than middle gaps (25 m2 a–1) and small gaps (11 m2 a–1) (Table 3). On the contrary, the size percentage of closure rate of small gaps (12%) was higher than middle gaps (9%) and large gaps (7%) (Table 3). The lateral growth rates of middle gaps and large gaps were similar (ca. 52 cm a–1), and were higher than small gaps (ca. 44 cm a–1) (Table 3).
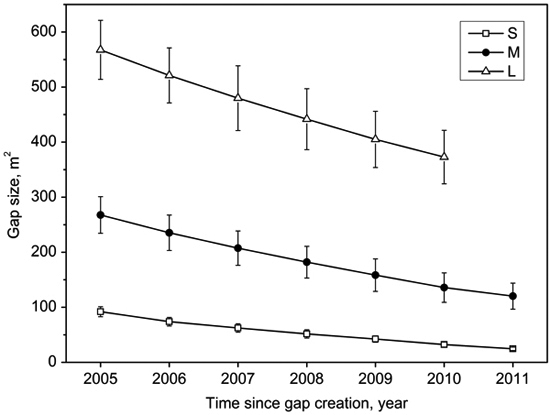
Fig. 2. Mean (with standard error) size of small gaps (S), middle gaps (M), and large gaps (L) as a function of time after gap creation in December 2004.
| Table 2. Gap closure equations of lateral extension growth for small gaps (S), middle gaps (M) and large gaps (L). | |
| Gap type | Canopy gap size (m2) |
| S | y = 0.7333x2 – 16.765x + 106.59 (n = 42) |
| M | y = 1.3584x2 – 35.521x + 301.61 (n = 28) |
| L | y = 1.5937x2 – 50.018x + 615.56 (n = 12) |
| x = gap age y = gap size n = number of hemispherical photographs | |
| Table 3. Mean (with standard error) closure rates and lateral extension growth rates of small gaps (S), middle gaps (M) and large gaps (L) for seven (six for large gaps) years. | |||
| Gap type | Closure rate (m2 a–1) | Percentage of closure rate (% a–1) | Lateral growth rate (cm a–1) |
| S | 11.2 ± 1.5 | 12.2 ± 1.6 | 44.4 ± 3.3 |
| M | 24.5 ± 2.3 | 9.2 ± 0.9 | 51.8 ± 3.6 |
| L | 38.9 ± 2.4 | 6.9 ± 0.4 | 52.0 ± 3.7 |
Gap size showed significantly positive correlations with canopy openness (R2 = 0.84, p < 0.001; Fig. 3). The canopy openness in the forest understory fluctuated slightly during 7 years (Fig. 4). Canopy openness of large gaps and middle gaps were remarkably different from forest understory during the monitoring (Fig. 4). There was no significant difference (p > 0.05) between small gaps and forest understory since 2009 (Fig. 4), although the size of small gaps still decreased smoothly (Fig. 2).
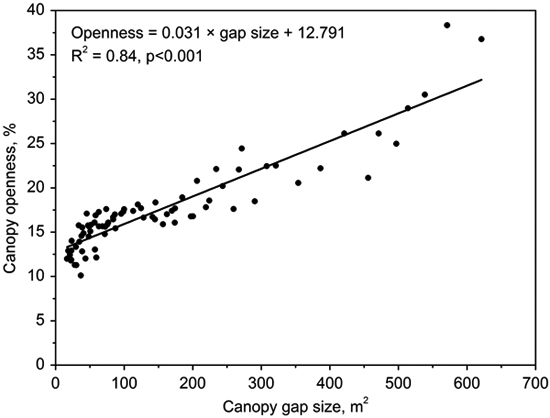
Fig. 3. Correlation between canopy openness and canopy gap size.
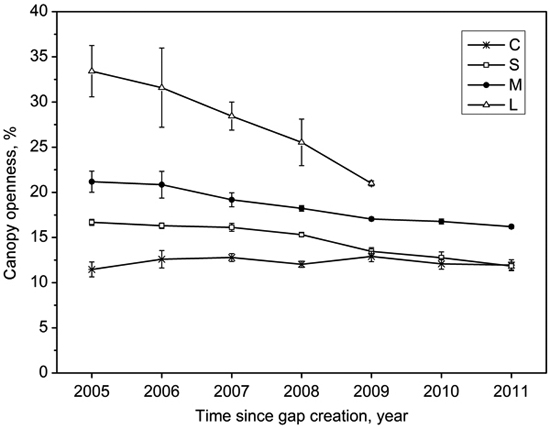
Fig. 4. Mean (with standard error) variation of canopy openness in the forest understory (C), small gaps (S), middle gaps (M), and large gaps (L) after gap creation in December 2004.
3.2 Woody species regeneration within the gaps
The density of regenerated woody species within gaps with different sizes was significantly higher than that in the forest understory in 2006 (i.e., two growing seasons after gap formation) (Fig. 5). In 2011, the density of regenerated woody species in all gaps decreased greatly, but the density in the forest understory kept stable (Fig. 5). Except for the large gaps, the density showed no significant differences between small gaps, middle gaps and the intact forest understory (Fig. 5). In 2006, the volume of regenerated woody species in small gaps did not significantly differ from that in the forest understory, but the volume in middle gaps and large gaps were significantly higher than that in the forest understory (Fig. 5). After 5 years, the volume of regenerated woody species increased significantly from forest understory, small gaps, middle gaps to large gaps (Fig. 5). Both density and volume in large gaps were predominant during the monitoring years (Fig. 5).
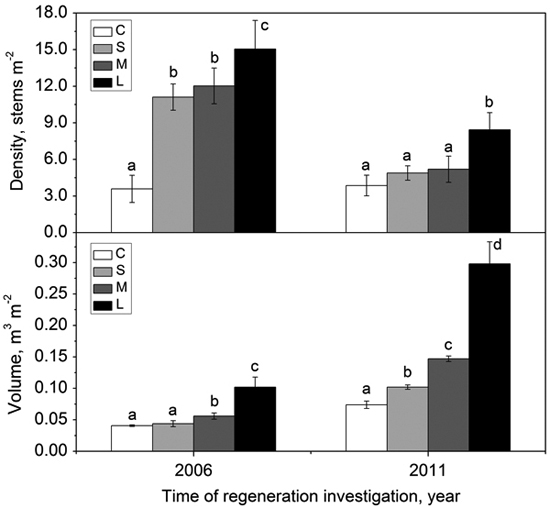
Fig. 5. Density (with standard error) and volume (with standard error) of woody species regeneration in the forest understory (C), small gaps (S), middle gaps (M), and large gaps (L) in 2006 and 2011 after gap creation (December 2004). Bars with different letters are significantly different (p < 0.05).
4 Discussion
We found that the lateral extension growth of the trees surrounding gaps played a predominant role in the closure process of all canopy gaps based on the observation of the first seven years of regeneration. The gap closure rates can be modeled by quadratic function curves (Table 2), i.e., the closure rates slowed down with gap ages, which is in agreement with previous observations by both van der Meer and Bongers (1996) and Valverde and Silvertown (1997). After gap formation, additional growing space stimulated or accelerated the lateral extension growth of canopy trees surrounding gaps (Frelich and Martin 1988), but with the lateral extension growth the gap size gradually decreased and eventually recovered to the canopy similar to the original one. Thus this gap effect decreased with time, and reflected in decreasing gap closure rates recorded by consecutive hemispherical photographs. As expected, mean annual size reduction ranked from large gaps to small gaps in descending order (Table 3). Although the size reduction of large gaps was significantly higher than middle gaps and small gaps (39, 25 versus 11 m2 a–1), closure percentage showed reverse patterns (7, 9 versus 12%), which were due to larger original size values. The canopy openness of small gaps did not significantly differ from that of the forest understory since 2009, but the gap size still decreased slowly afterwards. Thus, judging gap closure based on comparing the canopy openness may have risk of underestimating gap closure time.
Previous studies usually estimated gap closure process by supposing a constant lateral extension growth rate (Rentch et al. 2003) because continuously long term monitoring is not easy (Bekker et al. 2007). However, this assumption may not be realistic (Webster and Lorimer 2005) because the decrease of gap size due to lateral extension growth could not continue indefinitely (Ogden et al. 1991). Resource availabilities, mainly light and space, which are the limiting factors, decrease with time. Besides dynamics of resource conditions, tree architecture and stem density limit its indefinitely lateral extension growth (Alves and Santos 2002; Lida et al. 2012). This may be one reason why some large gaps without understory regeneration could exist decades (Lertzman and Krebs 1991). We estimated the deadline of lateral extension growth and corresponding remaining gap size by using quadratic functions established on the gap monitoring of consecutive years. Mean lateral growth rates of gaps with different sizes decreased yearly. The lateral extension growth of small gaps could approximately last 11 years (when the derivative of quadratic function equals zero; with a remaining gap size of 12 m2), middle gaps could last 13 years (with a remaining gap size of 69 m2), and large gaps could last 16 years (with a remaining gap size of 223 m2). We found that the mean lateral extension growth rate (48 cm a–1, varied from 13 cm a–1 to 79 cm a–1) in our study area was great higher than sub-alpine coniferous forests and some other temperate broad-leaved forests, but lower than tropical rain forests. For example, Ogden et al. (1991) claimed that canopy gaps with a radius of 7 m in a sub-alpine forest predominated by coniferous species Dacrydium biforme (Hook.) Pilg. and Phyllocladus alpinus Hook. would take more than 100 years to close given the same lateral extension growth rate. Runkle and Yetter (1987) pointed out that the mean lateral extension growth rate was 18 cm a–1 with a maximum rate of 59 cm a–1 in old-growth temperate forests. While van der Meer and Bongers (1996) reported that canopy gaps with a radius of 3 m in a tropical rain forest closed through lateral extension growth after 5–6 years (a mean growth rate of 55 cm a–1). The difference may be explained by the fact that: (1) Different climate zones provide different growth environment for tree species. Trees usually grow faster in tropical rain forests. (2) Forest age and gap age affect tree growth. The growth rates in old-growth forests gradually level off and slow down, but probably accelerate in relatively young secondary-growth forests (Rentch et al. 2003). Similarly, older gaps exhibit lower lateral extension growth rates, but the growth rates of new gaps are relatively higher (van der Meer and Bongers 1996). (3) Stem density of canopy trees influences lateral extension growth rates. Trees in lower stem density are more inclined to grow through lateral extension compared with trees in higher stem density. (4) Species of trees surrounding gaps affect the lateral extension growth rates. The monopodial crown shape of conifers, such as Pinus, Picea and Abies, limits their ability of lateral extension growth compared with broad-leaved species (Lertzman and Krebs 1991; Bartemucci et al. 2002). (5) It is also reported that low wood density species prefer vertical stem growth, while high woody density species prefer lateral extension growth (Lida et al. 2011). This partly explained our results of higher lateral extension growth rates, because the wood densities of Fraxinus rhynchophylla, Acer mono, and Quercus mongolica are relatively higher.
Generally, woody species regeneration within gaps showed difference from that in the forest understory during the observation period. The seedling density within gaps dramatically increased in 2 years after gap formation due to higher light intensity, soil moisture and growing space, but the volume of regenerated seedlings did not have much increase because the newly recruited individuals were small in size, though large in number. Micro-site conditions recovered to pre-gap state gradually with the gap size decreased (Kern et al. 2012). This environmental variation could be reflected in the decreasing seedling density within gaps. After 7 years, only large gaps still kept a higher density relative to the forest understory (Fig. 5) because a remaining size >300 m2 (Table 2) may provide sufficient resource availability for the regenerated seedlings (Denslow 1987). The volume of regenerated seedlings showed an increasing trend from small gaps to large gaps, and they all significantly higher than that in the forest understory (Fig. 5). After species competition, a few winners survived and experienced growth release (Bernal et al. 2012), which may lead to a decreasing density but increasing volume (Hart and Grissino-Mayer 2009). These findings support the previous reports that gaps promote regeneration in early stage (Hökkä et al. 2011; Zhu et al. 2014a).
However, continuous monitoring is still needed to determine how regeneration develops in response to gradually decreasing gap sizes. Hart and Grissino-Mayer (2009) investigated the regeneration within gaps in a secondary hardwood forest, and found that Acer saccharum Marshall, Acer rubrum L., Liriodendron tulipifera L., and Fagus grandifolia Ehrh. were the mainly regenerated species, even though their shade tolerance varied from shade-tolerant to shade-intolerant. They estimated that regeneration in a mean expanded gap size of 285 m2 might succeed, otherwise gaps would probably close via lateral extension growth. After 7 year’s study, we found that nearly all regenerated woody species showed positive responses to gaps (Tables 4, 5). However, density of shade-intolerant species usually exhibited an increasing trend from forest understory to large gaps (Fraxinus rhynchophylla, etc.), while density of shade-tolerant species was sometimes highest in middle (Acer pseudo-sieboldianum (Pax) Komarov, etc.) or small gaps (Ulmus laciniata (Trautv.) Mayr, etc.). On one hand, our results confirmed the positive effects of gaps on woody species regeneration in the early stage (Denslow 1987; Zhu et al. 2014a). On the other hand, our results also supported previous studies which reported that different gap sizes resulted in differences of woody species regeneration (Runkle 1982; Denslow 1987; Kern et al. 2013). According to the quadratic equation, lateral extension growth could last 16 years for large gaps, and eventually leave an opening of 223 m2 for seedlings and saplings growing into the canopy layer (Table 2). Thus, we suppose that the regeneration within large gaps may succeed, especially for the shade-intolerant species. However, the regeneration in small gaps might fail in the long run. Although a size of 25 m2 still remained after 7 years, the size would decrease to 12 m2 after 11 years. This small opening may not be regarded as a gap according to the lower limit of gap size, which is objectively defined by the mean shadow length of canopy trees surrounding the gap (Zhu et al. 2015), and not be enough for woody species growing up to canopy layer without repeated disturbances (Bernal et al. 2012). This result was similar to other studies such as Arevalo and Fernandez-Palacios (1998) and (2007). They investigated the regenerated species (a total of 13 species) including different shade tolerance dominated by Laurus azorica (Seub.) Franco and Prunus lusitanica L. in the laurel forest of Tenerife, Canary Islands, and pointed out that regeneration in gaps with size less than 100 m2 was insignificantly different from regeneration below the canopy. Middle gaps sometimes acted as “optimal size range” for species regeneration (Dumais and Prévost 2014). For example, after 5-year study, Dumais and Prévost (2014) claimed that 100–300 m2 canopy gaps provided favorable micro-environmental conditions which promoted the growth of red spruce (Picea rubens Sarg.). However, Arevalo and Fernandez-Palacios (2007) argued that gaps between 100 and 300 m2 inhibited the regeneration, because increased light intensity was insufficient for light-demanding species, but superfluous for shade-tolerant species. We found that the regeneration of most species in middle gaps (150–350 m2) fell in between small gaps and large gaps in the 7th year after gap formation (Tables 4, 5; Fig. 5). Shade-intolerant species in middle gaps grew better than in small gaps, and shade-tolerant species in middle gaps grew better than in large gaps. In addition, the size of middle gaps would keep stable at 69 m2 after 13 years. Thus, we infer that middle gaps provide a relatively ideal growth environment for the regeneration of most woody species in the temperate secondary forest, and saplings within middle gaps might finally reach the canopy height.
| Table 4. Density of regenerated woody species in the forest understory (C), small gaps (S), middle gaps (M), and large gaps (L) in 2006 and 2011. | |||||||||
| Species | Shade tolerance | Density (stems m–2) | |||||||
| 2006 | 2011 | ||||||||
| C | S | M | L | C | S | M | L | ||
| Betula costata Trautv. | shade-intolerant | 0.13 | 0.32 | 0.60 | 0.99 | 0.10 | 0.11 | 0.25 | 0.77 |
| Fraxinus mandshurica Rupr. | shade-intolerant | 0.32 | 0.99 | 1.09 | 1.46 | 0.46 | 0.46 | 0.47 | 1.02 |
| Fraxinus rhynchophylla | shade-intolerant | 0.77 | 1.96 | 2.48 | 3.66 | 0.58 | 0.73 | 0.75 | 1.58 |
| Juglans mandshurica Maxim. | shade-intolerant | 0.02 | 0.01 | 0.09 | 0.07 | 0.01 | 0.00 | 0.08 | 0.06 |
| Phellodendron amurense Rupr. | shade-intolerant | 0.09 | 0.51 | 0.67 | 0.83 | 0.09 | 0.20 | 0.23 | 0.64 |
| Populus davidiana Dode | shade-intolerant | 0.23 | 0.67 | 1.70 | 2.30 | 0.15 | 0.14 | 0.58 | 1.36 |
| Quercus mongolica | shade-intolerant | 0.98 | 1.08 | 1.43 | 1.82 | 0.76 | 0.34 | 0.71 | 1.02 |
| Alnus sibirica (Spach) Turcz. | intermediate | 0.24 | 0.43 | 0.61 | 0.20 | 0.30 | 0.16 | 0.29 | 0.06 |
| Aralia elata (Miq.) Seem. | intermediate | 0.00 | 0.58 | 0.54 | 0.79 | 0.00 | 0.13 | 0.21 | 0.31 |
| Acer mono | shade-tolerant | 0.43 | 1.17 | 1.20 | 1.49 | 0.61 | 0.56 | 0.80 | 0.77 |
| Acer pseudo-sieboldianum | shade-tolerant | 0.20 | 0.97 | 0.44 | 0.93 | 0.46 | 0.68 | 0.20 | 0.43 |
| Acer ukurunduense Trautv. | shade-tolerant | 0.06 | 0.67 | 0.41 | 0.00 | 0.10 | 0.59 | 0.12 | 0.00 |
| Corylus mandshurica Maxim. | shade-tolerant | 0.11 | 0.92 | 0.21 | 0.00 | 0.14 | 0.44 | 0.20 | 0.00 |
| Tilia amurensis Rupr. | shade-tolerant | 0.00 | 0.22 | 0.42 | 0.51 | 0.00 | 0.10 | 0.20 | 0.40 |
| Ulmus laciniata | shade-tolerant | 0.00 | 0.61 | 0.14 | 0.00 | 0.10 | 0.25 | 0.10 | 0.00 |
| Table 5. Volume of regenerated woody species in the forest understory (C), small gaps (S), middle gaps (M), and large gaps (L) in 2006 and 2011. | |||||||||
| Species | Shade tolerance | Volume (m3 m–2) | |||||||
| 2006 | 2011 | ||||||||
| C | S | M | L | C | S | M | L | ||
| Betula costata | shade-intolerant | 0.002 | 0.001 | 0.002 | 0.004 | 0.003 | 0.003 | 0.007 | 0.018 |
| Fraxinus mandshurica | shade-intolerant | 0.004 | 0.004 | 0.004 | 0.007 | 0.007 | 0.012 | 0.014 | 0.039 |
| Fraxinus rhynchophylla | shade-intolerant | 0.004 | 0.003 | 0.004 | 0.007 | 0.006 | 0.006 | 0.006 | 0.014 |
| Juglans mandshurica | shade-intolerant | 0.003 | 0.004 | 0.007 | 0.015 | 0.007 | 0.006 | 0.013 | 0.034 |
| Phellodendron amurense | shade-intolerant | 0.002 | 0.003 | 0.005 | 0.012 | 0.003 | 0.003 | 0.008 | 0.024 |
| Populus davidiana | shade-intolerant | 0.003 | 0.002 | 0.003 | 0.007 | 0.003 | 0.003 | 0.007 | 0.019 |
| Quercus mongolica | shade-intolerant | 0.002 | 0.001 | 0.002 | 0.005 | 0.003 | 0.003 | 0.007 | 0.016 |
| Alnus sibirica | Intermediate | 0.006 | 0.004 | 0.005 | 0.012 | 0.008 | 0.010 | 0.012 | 0.030 |
| Aralia elata | Intermediate | 0.000 | 0.004 | 0.006 | 0.015 | 0.000 | 0.009 | 0.018 | 0.039 |
| Acer mono | shade-tolerant | 0.005 | 0.003 | 0.002 | 0.006 | 0.007 | 0.007 | 0.007 | 0.018 |
| Acer pseudo-sieboldianum | shade-tolerant | 0.003 | 0.003 | 0.002 | 0.005 | 0.007 | 0.008 | 0.008 | 0.017 |
| Acer ukurunduense | shade-tolerant | 0.003 | 0.003 | 0.002 | 0.000 | 0.007 | 0.007 | 0.007 | 0.000 |
| Corylus mandshurica | shade-tolerant | 0.004 | 0.004 | 0.004 | 0.000 | 0.007 | 0.010 | 0.012 | 0.000 |
| Tilia amurensis | shade-tolerant | 0.000 | 0.001 | 0.002 | 0.006 | 0.000 | 0.004 | 0.011 | 0.028 |
| Ulmus laciniata | shade-tolerant | 0.000 | 0.003 | 0.004 | 0.000 | 0.006 | 0.010 | 0.011 | 0.000 |
Gap sizes affect the gap duration. Large gaps had higher closure rates but lower closure percentages relative to small gaps. Gaps, especially large ones, significantly promoted woody species regeneration in the early stage, but this promotion gradually weakened with time. Thus, large gaps may provide more opportunities for filling regeneration in the long term because of longer gap duration. Middle gaps provide appropriate growth environment for most woody species. However, the regeneration in small gaps may eventually fail without addition gap disturbance. Considering the sample size of large gaps (2 repetitions), middle gaps (4 repetitions) and small gaps (6 repetitions) in our study, it may be acceptable in experiments of artificial gaps (Dumais and Prévost 2014). On one hand, we had to be subject to the conservation policy of natural forests, which strictly limited the cutting area. On the other hand, although the sample size was small, we selected similar environmental conditions such as slope, aspect, altitude, and tree species composition, and created artificial gaps instead of using natural gaps for better controlling and unifying the initial environmental conditions and gap characteristics. The homogeneity of environment allowed us to minimize the random errors, and distinguish the effects of gap size on lateral extension growth of canopy trees surrounding gaps and regeneration within gaps. Thus, our results were comparable not only within our study, but also with other similar studies. Our goal is to detect the detailed gap closure process of different sizes. Continuously long term monitoring with short term interval of lateral extension growth and regeneration information of different species are needed to test the speculation, and provide more robust evidence of appropriate gap size for successful regeneration in temperate secondary forests.
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (31330016), and the National Basic Research Program of China (2012CB416906). We thank Dr. G. Geoff Wang from school of Agricultural, Forest and Environmental Sciences, Clemson University, and Dr. Tian Gao from Institute of Applied Ecology, Chinese Academy of Sciences for their helpful discussion during the study. We also thank the chief editor of Silva Fennica Dr. Eeva Korpilahti and the three anonymous reviewers for their valuable criticisms, suggestions and the detail revisions on our manuscript.
References
Alves L.F., Santos F.A.M. (2002). Tree allometry and crown shape of four tree species in Atlantic rain forest, south-east Brazil. Journal of Tropical Ecology 18: 245–260. http://dx.doi.org/10.1017/S026646740200216X.
Amir A.A. (2012). Canopy gaps and the natural regeneration of Matang mangroves. Forest Ecology and Management 269: 60–67. http://dx.doi.org/10.1016/j.foreco.2011.12.040.
Andersen H.E., Reutebuch S.E., McGaughey R.J., d’Oliveira M.V.N., Keller M. (2014). Monitoring selective logging in western Amazonia with repeat lidar flights. Remote Sensing of Environment 151: 157–165. http://dx.doi.org/10.1016/j.rse.2013.08.049.
Arevalo J.R., Fernandez-Palacios J.M. (1998). Treefall gap characteristics and regeneration in the laurel forest of Tenerife. Journal of Vegetation Science 9: 297–306. http://dx.doi.org/10.2307/3237094.
Arevalo J.R., Fernandez-Palacios J.M. (2007). Treefall gaps and regeneration composition in the laurel forest of Anaga (Tenerife): a matter of size? Plant Ecology 188: 133–143. http://dx.doi.org/10.1007/s11258-006-9152-1.
Bartemucci P., Coates K.D., Harper K.A., Wright E.F. (2002). Gap disturbances in northern old-growth forests of British Columbia, Canada. Journal of Vegetation Science 13: 685–696. http://dx.doi.org/10.1111/j.1654-1103.2002.tb02096.x.
Bekker R.M., van der Maarel E., Bruelheide H., Woods K. (2007). Long-term datasets: From descriptive to predictive data using ecoinformatics. Journal of Vegetation Science 18: 458–462. http://dx.doi.org/10.1658/1100-9233(2007)18[458:ldfdtp]2.0.co;2.
Bernal P.M.L., Defosse G.E., Quinteros C.P., Bava J.O. (2012). Sapling growth and crown expansion in canopy gaps of Nothofagus pumilio (lenga) forests in Chubut, Patagonia, Argentina. Forest Systems 21: 489–497. http://dx.doi.org/10.5424/fs/2012213-02538.
Brokaw N.V.L. (1985). Gap-phase regeneration in a tropical forest. Ecology 66: 682–687. http://dx.doi.org/10.2307/1940529.
Brokaw N., Busing R.T. (2000). Niche versus chance and tree diversity in forest gaps. Trends in ecology & evolution 15: 183–188. http://dx.doi.org/10.1016/s0169-5347(00)01822-x.
Brusa A., Bunker D.E. (2014). Increasing the precision of canopy closure estimates from hemispherical photography: Blue channel analysis and under-exposure. Agricultural and Forest Meteorology 195: 102–107. http://dx.doi.org/10.1016/j.agrformet.2014.05.001.
Chazdon R.L. (1986). Light variation and carbon gain in rain-forest understorey palms. Journal of Ecology 74: 995–1012. http://dx.doi.org/10.2307/2260229.
Denslow J.S. (1987). Tropical rainforest gaps and tree species diversity. Annual review of ecology and systematics 18: 431–451. http://dx.doi.org/10.1146/annurev.ecolsys.18.1.431.
Dumais D., Prévost M. (2014). Physiology and growth of advance Picea rubens and Abies balsamea regeneration following different canopy openings. Tree physiology 34: 194–204. http://dx.doi.org/10.1093/treephys/tpt114.
Dupuy J.M., Chazdon R.L. (2008). Interacting effects of canopy gap, understory vegetation and leaf litter on tree seedling recruitment and composition in tropical secondary forests. Forest Ecology and Management 255: 3716–3725. http://dx.doi.org/10.1016/j.foreco.2008.03.021.
Forrester J.A., Lorimer C.G., Dyer J.H., Gower S.T., Mladenoff D.J. (2014). Response of tree regeneration to experimental gap creation and deer herbivory in north temperate forests. Forest Ecology and Management 329: 137–147. http://dx.doi.org/10.1016/j.foreco.2014.06.025.
Frazer G.W., Canham C.D., Lertzman K.P. (1999). Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Copyright © 1999: Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York. http://www.caryinstitute.org/science-program/our-scientists/dr-charles-d-canham/gap-light-analyzer-gla.
Frazer G.W., Fournier R.A., Trofymow J.A., Hall R.J. (2001). A comparison of digital and film fisheye photography for analysis of forest canopy structure and gap light transmission. Agricultural and Forest Meteorology 109: 249–263. http://dx.doi.org/10.1016/s0168-1923(01)00274-x.
Frelich L.E., Martin G.L. (1988). Effects of crown expansion into gaps on evaluation of disturbance intensity in northern hardwood forests. Forest Science 34: 530–536.
Getzin S., Nuske R.S., Wiegand K. (2014). Using unmanned aerial vehicles (UAV) to quantify spatial gap patterns in forests. Remote Sens-Basel 6: 6988–7004. http://dx.doi.org/10.3390/Rs6086988.
Gutiérrez A.G., Aravena J.C., Carrasco-Farías N.V., Christie D.A., Fuentes M., Armesto J.J. (2008). Gap-phase dynamics and coexistence of a long-lived pioneer and shade-tolerant tree species in the canopy of an old-growth coastal temperate rain forest of Chiloé Island, Chile. Journal of Biogeography 35: 1674–1687. http://dx.doi.org/10.1111/j.1365-2699.2008.01908.x.
Hart J.L., Grissino-Mayer H.D. (2009). Gap-scale disturbance processes in secondary hardwood stands on the Cumberland Plateau, Tennessee, USA. Plant Ecology 201: 131–146. http://dx.doi.org/10.1007/s11258-008-9488-9.
Hartshorn G.S. (1989). Application of gap theory to tropical forest management: natural regeneration on strip clear-cuts in the Peruvian Amazon. Ecology 70: 567–569. http://dx.doi.org/10.2307/1940208.
Hökkä H., Mäkelä H. (2014). Post-harvest height growth of Norway spruce seedlings in northern Finland peatland forest canopy gaps and comparison to partial and complete canopy removals and plantations. Silva Fennica 48. http://dx.doi.org/10.14214/sf.1192.
Hökkä H., Repola J., Moilanen M., Saarinen M. (2011). Seedling survival and establishment in small canopy openings in drained spruce mires in Northern Finland. Silva Fennica 45: 633–645. http://dx.doi.org/10.14214/sf.97.
Hu L.L., Gong Z.W., Li J.S., Zhu J.J. (2009). Estimation of canopy gap size and gap shape using a hemispherical photograph. Trees-Structure and Function 23: 1101–1108. http://dx.doi.org/10.1007/s00468-009-0353-9.
Jelaska S.D., Antonic O., Bozic M., Krizan J., Kusan V. (2006). Responses of forest herbs to available understory light measured with hemispherical photographs in silver fir-beech forest in Croatia. Ecological Modelling 194: 209–218. http://dx.doi.org/10.1016/j.ecolmodel.2005.10.013.
Kathke S., Bruelheide H. (2010). Gap dynamics in a near-natural spruce forest at Mt. Brocken, Germany. Forest Ecology and Management 259: 624–632. http://dx.doi.org/10.1016/j.foreco.2009.11.021.
Kern C.C., Reich P.B., Montgomery R.A., Strong T.F. (2012). Do deer and shrubs override canopy gap size effects on growth and survival of yellow birch, northern red oak, eastern white pine, and eastern hemlock seedlings? Forest Ecology and Management 267: 134–143. http://dx.doi.org/10.1016/j.foreco.2011.12.002.
Kern C.C., D’Arnato A.W., Strong T.F. (2013). Diversifying the composition and structure of managed, late-successional forests with harvest gaps: What is the optimal gap size? Forest Ecology and Management 304: 110–120. http://dx.doi.org/10.1016/j.foreco.2013.04.029.
Kobe R.K., Hogarth L.J. (2007). Evaluation of irradiance metrics with respect to predicting sapling growth. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 37: 1203–1213. http://dx.doi.org/10.1139/X06-320.
Koivula M., Kuuluvainen T., Hallman E., Kouki J., Siitonen J., Valkonen S. (2014). Forest management inspired by natural disturbance dynamics (DISTDYN) - a long-term research and development project in Finland. Scandinavian Journal of Forest Research 29: 579–592. http://dx.doi.org/10.1080/02827581.2014.938110.
Lertzman K.P., Krebs C.J. (1991). Gap-phase structure of a subalpine old-growth forest. Canadian Journal of Forest Research 21: 1730–1741. http://dx.doi.org/10.1139/x91-239.
Lida Y., Poorter L., Sterck F.J., Kassim A.R., Kubo T., Potts M.D., Kohyama T.S. (2012). Wood density explains architectural differentiation across 145 co-occurring tropical tree species. Functional Ecology 26: 274–282. http://dx.doi.org/10.1111/j.1365-2435.2011.01921.x.
Long J.N. (2009). Emulating natural disturbance regimes as a basis for forest management: A North American view. Forest Ecology and Management 257: 1868–1873. http://dx.doi.org/10.1016/j.foreco.2008.12.019.
Ogden J., Fordham R.A., Pilkington S., Serra R.G. (1991). Forest gap formation and closure along an altitudinal gradient in Tongariro National Park, New Zealand. Journal of Vegetation Science 2: 165–172. http://dx.doi.org/10.2307/3235948.
Rentch J.S., Fajvan M.A., Hicks Jr R.R. (2003). Oak establishment and canopy accession strategies in five old-growth stands in the central hardwood forest region. Forest Ecology and Management 184: 285–297. http://dx.doi.org/10.1016/S0378-1127(03)00155-5.
Runkle J.R. (1982). Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 63: 1533–1546. http://dx.doi.org/10.2307/1938878.
Runkle J.R., Yetter T.C. (1987). Treefalls revisited: gap dynamics in the Southern Appalachians. Ecology 68: 417–424. http://dx.doi.org/10.2307/1939273.
Schliemann S.A., Bockheim J.G. (2011). Methods for studying treefall gaps: a review. Forest ecology and management 261: 1143–1151. http://dx.doi.org/10.1016/j.foreco.2011.01.011.
Schnitzer S.A., Carson W.P. (2001). Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 82: 913–919. http://dx.doi.org/10.2307/2679891.
Schumann M.E., White A.S., Witham J.W. (2003). The effects of harvest-created gaps on plant species diversity, composition, and abundance in a Maine oak-pine forest. Forest Ecology and Management 176: 543–561. http://dx.doi.org/10.1016/s0378-1127(02)00233-5.
Valverde T., Silvertown J. (1997). Canopy closure rate and forest structure. Ecology 78: 1555–1562. http://dx.doi.org/10.2307/2266148.
van der Meer P.J., Bongers F. (1996). Formation and closure of canopy gaps in the rain forest at Nouragues, French Guiana. Vegetatio 126: 167–179. http://dx.doi.org/10.1007/bf00045602.
Vepakomma U., Kneeshaw D., Fortin M.J. (2012). Spatial contiguity and continuity of canopy gaps in mixed wood boreal forests: persistence, expansion, shrinkage and displacement. Journal of Ecology 100: 1257–1268. http://dx.doi.org/10.1111/j.1365-2745.2012.01996.x.
Vilhar U., Roženbergar D., Simončič P., Diaci J. (2014). Variation in irradiance, soil features and regeneration patterns in experimental forest canopy gaps. Annals of Forest Science. http://dx.doi.org/10.1007/s13595-014-0424-y.
Wang G.L., Liu F. (2011). The influence of gap creation on the regeneration of Pinus tabuliformis planted forest and its role in the near-natural cultivation strategy for planted forest management. Forest Ecology and Management 262: 413–423. http://dx.doi.org/10.1016/j.foreco.2011.04.007.
Watt A.S. (1947). Pattern and process in the plant community. Journal of Ecology 35: 1–22. http://dx.doi.org/10.2307/2256497.
Weber T.A., Hart J.L., Schweitzer C.J., Dey D.C. (2014). Influence of gap-scale disturbance on developmental and successional pathways in Quercus-Pinus stands. Forest Ecology and Management 331: 60–70. http://dx.doi.org/10.1016/j.foreco.2014.08.006.
Webster C.R., Lorimer C.G. (2005). Minimum opening sizes for canopy recruitment of midtolerant tree species: A retrospective approach. Ecological Applications 15: 1245–1262. http://dx.doi.org/10.1890/04-0763.
Whitmore T. (1989). Canopy gaps and the two major groups of forest trees. Ecology 70: 536–538. http://dx.doi.org/10.2307/1940195.
Yamamoto S.I. (2000). Forest gap dynamics and tree regeneration. Journal of Forest Research 5: 223–229. http://dx.doi.org/10.1007/BF02767114.
Yan Q.L., Zhu J.J., Yu L.Z. (2012). Seed regeneration potential of canopy gaps at early formation stage in temperate secondary forests, Northeast China. Plos One 7: e39502. http://dx.doi.org/10.1371/journal.pone.0039502.
York R.A., Heald R.C., Battles J.J., York J.D. (2004). Group selection management in conifer forests: relationships between opening size and tree growth. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 34: 630–641. http://dx.doi.org/10.1139/x03-222.
Young T.P., Hubbell S.P. (1991). Crown asymmetry, treefalls, and repeat disturbance of broad-leaved forest gaps. Ecology 72: 1464–1471. http://dx.doi.org/10.2307/1941119.
Zhang M., Zhu J.J., Li M.C., Zhang G.Q., Yan Q.L. (2013). Different light acclimation strategies of two coexisting tree species seedlings in a temperate secondary forest along five natural light levels. Forest Ecology and Management 306: 234–242. http://dx.doi.org/10.1016/j.foreco.2013.06.031.
Zhu J.J., Matsuzaki T., Lee F.Q., Gonda Y. (2003). Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. Forest Ecology and Management 182: 339–354. http://dx.doi.org/10.1016/s0378-1127(03)00094-x.
Zhu J.J., Mao Z.H., Hu L., Zhang J.X. (2007). Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. Journal of Forest Research 12: 403–416. http://dx.doi.org/10.1007/s10310-007-0033-9.
Zhu J.J., Lu D.L., Zhang W.D. (2014a). Effects of gaps on regeneration of woody plants: a meta-analysis. Journal of Forestry Research 25: 501–510. http://dx.doi.org/10.1007/s11676-014-0489-3.
Zhu J.J., Wang K., Sun Y.R., Yan Q.L. (2014b). Response of Pinus koraiensis seedling growth to different light conditions based on the assessment of photosynthesis in current and one-year-old needles. Journal of Forestry Research 25: 53–62. http://dx.doi.org/10.1007/s11676-014-0432-7.
Zhu J.J., Zhang G.Q., Wang G.G., Yan Q.L., Lu D.L., Li X.F., Zheng X. (2015). On the size of forest gaps: can their lower and upper limits be objectively defined? Agriculture and Forest Meteorology 213: 64–76. http://dx.doi.org/10.1016/j.agrformet.2015.06.015.
Total of 61 references.

