Comparison of damage risks in even- and uneven-aged forestry in Finland
Nevalainen S. (2017). Comparison of damage risks in even- and uneven-aged forestry in Finland. Silva Fennica vol. 51 no. 3 article id 1741. https://doi.org/10.14214/sf.1741
Highlights
- Damage risks in two forest management regimes were estimated by means of a literature review and a questionnaire to Finnish forestry experts
- Damage risks were usually estimated to be higher in even-aged than in uneven-aged management regimes
- In some cases, however, damage risks may be higher in uneven-aged stands (root-rot infected Norway spruce stands and mechanical damage due to repeated thinnings).
Abstract
The literature on the most prominent forest damage related to even-aged and uneven-aged forest management regimes was reviewed. A questionnaire to expert researchers was conducted to estimate risks in even-aged and uneven-aged forest management chains in Finland. There are only a few empirical comparisons of damage risks in even- and uneven-aged stands in the literature. The results from the expert survey showed that the damage risks were higher in even-aged management in Norway spruce and Scots pine. However, the variation in the risks between individual chains and between individual causes was high. The highest risks in Scots pine were caused by moose (in even-aged chains) and harvesting damage (in uneven-aged chains). In Norway spruce, root rot caused the highest risks in both even-aged and uneven-aged chains. The higher risks in even-aged forestry are largely due to the many associated practices which favour various types of damage. However, there are some important exceptions: the damage risks may be higher in some uneven-aged stands, especially in Norway spruce stands infected with root rot where the utilization of undergrowth or natural regeneration can be risky. Moreover, the repeated thinnings in uneven-aged stands may lead to increased mechanical damage.
Keywords
forest damage;
uneven-aged forestry;
even-aged forestry
Received 9 December 2016 Accepted 18 April 2017 Published 11 May 2017
Views 285841
Available at https://doi.org/10.14214/sf.1741 | Download PDF

1 Introduction
Forestry is facing several challenges, not only increasing demand of timber and fuelwood, but also demands for maintaining biological diversity, carbon sequestration, protection of water resources, erosion control and recreational amenities (Diaci et al. 2011). One of the objectives of future forest management should be maintaining healthy forest ecosystems for maximum resilience, i.e., the ability to resist disturbance and the capability of returning to a stable state after disturbance. This is crucially important for adapted silviculture in the future, because of the predicted increase in damage risks under the changing climate (La Porta et al. 2006; Björkman et al. 2015; Subramanian et al. 2016). Therefore, forest management strategies should also be evaluated for their potential damage risks.
Even-aged, or rotation forest management (RFM), has been the most commonly applied forest management strategy in Europe. In Finland, for example, this type of forestry has been practised since the early 1950s, with a strong tendency to favour conifers. Typically, even-aged forestry includes clearcuts or strip-fellings, soil preparation, active regeneration, thinning of seedling stands, and several low thinnings (thinnings from below) of the growing stock.
Interest in alternative forest management regimes has been growing since the 1990s (Jonsson et al. 2011). These management options are called uneven-aged forestry, continuous cover management or clearcut-free management in Europe. Uneven-aged forestry mostly relies on patch harvesting (small clearcuts), selective thinnings (high thinnings, thinnings from above) and natural regeneration or utilization of the undergrowth. One of the basic ideas behind uneven-aged forest management is “natural disturbance management”, i.e., an attempt to emulate the size, severity and quality of natural disturbances to maintain the natural-like structure of forests (Kuuluvainen and Grenfell 2012). Stands in the transformation stage (even-aged forests to be converted into continuous-cover forests) should also be considered in this context.
Uneven-aged or continuous cover forests may lead to increased habitat complexity, mostly because of increased structural diversity. To some extent, uneven-aged forests may also have higher compositional diversity (trees of different species growing in mixtures at the same site). For the scope of this study, functional diversity (Schmidt 1978) is a suitable term, where functional implies resistance against epidemics, covering the mechanisms by which forests may resist diseases and pests. This term may also be considered to include diversity in edaphic and micro- and macroclimatic factors.
Nordic forests can be considered as mostly natural or semi-natural, but not plantation forests, regardless of the management regime. The structure of managed forests in Finland, for instance, is claimed to have remained largely similar to that of natural forests, due to forest management based on the natural site type classification and the use of indigenous species in regeneration (Parviainen and Västilä 2011). Many natural forests in the northern hemisphere are, however, relatively simple ecosystems, as compared to tropical forests, and are, in theory, susceptible to large outbreaks of insect epidemics (FAO 2001).
Whatever management methods are applied, they inevitably have an effect – negative or positive – on the risks of abiotic, biotic or mechanical (man-made) damage. The affecting factors include tree species diversity, age structure, understorey structure, tree density, rotation length, thinning intensity and the operations applied in each management strategy (method of final cutting, soil preparation, regeneration method, etc.). The greatest single deterrent to disease or pest epidemics is, however, the amount of suitable and susceptible host material. In this context, even-aged forests may be potentially more susceptible to widespread epidemics, because homogeneous host material is readily available.
There is a vast amount of literature on various types of damage in different stages of even-aged forestry, and a full review is impossible here. However, there are only a few empirical comparisons of damage risks in even- and uneven-aged stands. Aspects of fungal and insect damage in Sweden in this context have been reported by Björkman et al. (2011). Due to the lack of empirical studies, simulation studies or models have often been applied to study variable aspects of damage susceptibility. Important conclusions about damage risks can also be drawn by observing the stand structures and the different phases (the applied operations) in the management chains.
The aim of this work is to review the literature on the most prominent forest damage related to even-aged and uneven-aged forest management regimes. The main emphasis is on the Finnish boreal forests. However, relevant literature from Scandinavian countries is also cited. Expert opinions, i.e., the results from a survey to Finnish experts concerning damage risks in these two management regimes, are also presented here.
2 Materials and methods
2.1 Questionnaire to Finnish experts
Finnish experts were asked their opinions of specified damage risks in a total of 31 even-aged and uneven-aged practical forestry operational chains in South and North Finland. The experts invited to participate were experts with experience and education in forest pathology/entomology and silviculture. Eleven experts were asked to participate, and eight responded.
The practical operation chains included 11 even-aged and 6 uneven-aged chains with Scots pine (Pinus sylvestris L.) as the main tree species and 8 even-aged and 6 uneven-aged chains with Norway spruce (Picea abies L. Karst.) as the main species. The survey was restricted to mineral soil sites only. All the phases (practical operations) of each chain were described to the respondents. These descriptions were based on the current recommendations for silvicultural and management practices in Finland (Äijälä et al. 2014) and on discussions with Finnish silviculture experts.
For each chain, the main tree species (Norway spruce or Scots pine), method of final cutting, soil preparation, method of regeneration, need for weed control, need for early cleaning of the seedling stand, thinning, possibility for growing mixed tree species, possible pruning and fertilization were listed (for a description of the chains, see Table 1). Twenty-one causes of damage were listed for chains with Scots pine as the main tree species and 16 causes for Norway spruce chains. For a list of the causes of damage analysed, see Tables 2a,b and 4.
| Table 1. Description of the operational chains in Finnish forestry used in the expert survey. Please not that not all the alternatives within a chain are shown. For instance, the possible mixed tree species, usage of of nursery seedlings, need for weeding, possible fertilization or cuttings for seed tree removal are not listed here. | |||||||
| Chain | Strategy | Part of Finland | Tree species | Cutting method | Site type | Soil preparation | Regeneration |
| 1 | even aged | south | pine | clearcut | xeric/sub-xeric | harrowing/scalping | sowing |
| 2 | even-aged | south | pine | clearcut | sub-xeric | mounding | planting |
| 3 | even-aged | south | pine | seedling tree felling | sub-xeric | harrowing | natural |
| 4 | even-aged | south | pine | clearcut | mesic | harrowing/scalping | planting |
| 5 | even-aged | south | spruce | clearcut | herb-rich | no | planting |
| 6 | even-aged | south | spruce | clearcut | herb-rich | mounding | planting |
| 7 | even-aged | south | spruce | strip felling | herb-rich | no | natural |
| 8 | even-aged | south | spruce | strip felling | herb-rich | harrowing | natural |
| 9 | even-aged | south | spruce | shelterwood felling | herb-rich | no | natural |
| 14 | even-aged | north | pine | clearcut | xeric | harrowing | sowing |
| 15 | even-aged | north | pine | seedling tree felling | xeric | no | natural |
| 16 | even-aged | north | pine | seedling tree felling | xeric | harrowing | natural |
| 17 | even-aged | north | pine | clearcut | mesic/ sub-xeric | mounding | planting |
| 18 | even-aged | north | pine | clearcut | mesic/sub-xeric | harrowing | sowing |
| 19 | even-aged | north | pine | clearcut | mesic | harrowing | planting |
| 20 | even-aged | north | pine | seedling tree felling | sub-xeric | harrowing | natural |
| 21 | even-aged | north | spruce | clearcut | herb-rich rich/mesic | mounding | planting |
| 22 | even-aged | north | spruce | strip felling | herb-rich | no | natural |
| 23 | even-aged | north | spruce | strip felling | herb-rich | harrowing | natural |
| 24 | uneven-aged | south | pine | patch felling | xeric | no/light scalping | natural |
| 25 | uneven-aged | south | pine | patch felling | sub-xeric | no/light scalping | natural |
| 26 | uneven-aged | south | pine | selection felling | mesic | no | undergrowth |
| 27 | uneven-aged | south | pine | selection felling | mesic | no | natural |
| 28 | uneven-aged | south | spruce | patch felling | herb-rich rich/mesic | no/light scalping | natural |
| 29 | uneven-aged | south | spruce | selection felling | herb-rich rich/mesic | no | undergrowth |
| 30 | uneven-aged | north | pine | selection felling | sub-xeric | no | natural |
| 31 | uneven-aged | north | pine | patch felling | mesic | no/light scalping | natural |
| 32 | uneven-aged | north | spruce | patch felling | herb-rich | no/light scalping | natural |
| 33 | uneven-aged | north | spruce | selection felling | herb-rich | no | undergrowth |
| 34 | uneven-aged | north | spruce | selection felling | mesic | no | undergrowth |
| 35 | uneven-aged | north | spruce | patch felling | mesic | no/light scalping | natural |
| 36 | uneven-aged | south | pine | selection felling | sub-xeric | no | natural |
The operational chain and the potential cause of damage formed the conceptual framework, against which the experts were asked to answer, based on their experience and on the literature, the following questions:
- (A) What is the probability and economic significance of a specified cause of damage in each chain?
- (B) Which operations within the chain increase the risk of a specified damage?
The experts were asked to focus on the whole chain in an imaginary, average forest compartment, during its whole rotation period. The surrounding forest stands and the landscape level were thus not considered.
The questionnaire was conducted as a Webropol survey on the Internet. Question A was presented as an x–y space, onto which the experts could freely mark their opinions about the probability (x-axis) and the economic significance (y-axis) of the specific damage cause. Question B was a multiple-choice selection, where the respondents could choose many alternatives by marking the appropriate checkboxes. Depending on the operational chain, the alternatives could include:
- soil preparation method (no soil preparation, harrowing, scalping, mounding);
- regeneration method (planting, sowing, natural regeneration, utilization of undergrowth);
- weed control, clearing of broadleaves, early thinning, thinning of the seedling stand;
- thinning;
- cutting method (clearcutting, strip felling, patch harvesting, selection harvesting, seed tree removal, shelterwood felling);
- possibility of growing mixed species or a second layer.
Only a few of the respondents filled in all the points in question A. The theoretical maximum number of chain × damage × respondent -combinations was 10 086 in question A, and 2296 (23%) of these were responded and remained in the analysis. Two respondents answered questions regarding only one of the two alternative management regimes, and two respondents answered questions regarding only one cause of damage . The chains with silver birch (Betula pendula Roth) as the main tree species were discarded from the analysis, owing to the small number of responses for these chains.
To overcome the problems in the resulting heterogeneous data, the results for question A were in subsequent analyses transformed into a variable named “scaled damage risk”, as follows: The original estimated probability value (x-value) and economic significance (y-value) were divided by the maximum value in all damage-chain cases given by each respondent. These scaled x- and y-values were multiplied by each other and further weighted by the number of answers obtained for each damage cause (the main tree species and the management regimes were separated). The comparison of damage risk by a single cause between management regimes was based on the scaled damage risk: if the risk values were over two times higher or lower, this depicted a higher or lower risk, respectively. Values five times higher or lower depicted a much higher or lower risk, respectively. The scaled risk of a single causal agent in a management chain was also classified as low, medium or high risk. These classes were based on percentiles of the scaled damage risks of a specific cause in all the chains: percentiles of 51–70 present a medium risk and percentiles over 70 present a high risk.
The combined effects of operational methods, site factor, causes of damage and cutting methods were summarized and visualized with a recursive partitioning and regression-tree analysis, using the rpart module in the rpart package (Therneau et al. 2015) in R (R Core Team 2015). By definition, recursive partitioning creates a decision tree that strives to correctly classify members of a population by splitting the population into sub-populations based on several dichotomous independent variables. In this case, the analysis separated the data into groups with different damage risk values.
3 Results
3.1 A literature review of the important damage risks regarding different forest management regimes (with special reference to Finland)
3.1.1 Heterobasidion root rot
Heterobasidion parviporum Niemelä & Korhonen and H. annosum (Fr.) Bref. sensu stricto spread primarily via basidiospores on fresh stumps and harvesting or root injuries, and vegetatively by mycelium from the root system to adjacent trees. For a review, see Korhonen and Stenlid (1998). In unmanaged forests stumps and wounds are mostly absent. Human activities thus have a very important contribution to an increased incidence of Heterobasidion root rot due to summer cutting operations and trunk and root injuries caused by logging machines (Isomäki and Kallio 1974).
Once established in a stand, these fungi may survive for centuries. In the next planted generation Heterobasidion root rot becomes evident after about ten years. The incidence of Heterobasidion root rot tends to increase in successive tree rotations (Holmsgaard et al. 1961; Bendz-Hellgren and Stenlid 1998; Piri and Korhonen 2001; Piri 2003; Piri and Korhonen 2007; Rönnberg et al. 2007).
In diseased stands the vegetative spread of Heterobasidion has been more common in thinned than in unthinned spruce stands. The rapid expansion of old Heterobasidion infections after a thinning operation may significantly contribute to the occurrence of root rot in the residual stand (Piri and Korhonen 2008). The growth rate in stump roots is two to three times higher than in roots of standing trees (Bendz-Hellgren et al. 1999). Stumps in thinned stands have been more susceptible to infection than stumps in clearcuts or in pre-commercially thinned stands (Bendz-Hellgren and Stenlid 1998). But even small infected stumps of pre-commercial thinnings in Norway spruce stands are capable of transferring the infection to neighbouring trees (Gunulf et al. 2013). The frequency of thinnings has in many studies been correlated with the development of rot both in Norway spruce and in Scots pine stands (Vollbrecht and Agestam 1995; Pettersson et al. 2003; Möykkynen and Miina 2002; Rönnberg et al. 2006).
Planted spruces have been much more frequently infected than naturally regenerated seedlings in Switzerland. Planting under shade had a favourable effect, presumably due to the slow development and retarded formation of the root contacts with the infection sources (Graber 1997). Stands previously used as fields or for grazing are particularly susceptible to root rot (Vollbrecht and Agestam 1995).
Uneven-aged spruce stands maintaining continuous spruce regeneration favour the secondary spread of H. parviporum from overstorey trees and stumps to the next spruce generation. H. parviporum has spread more frequently from the inoculated stumps to the neighbouring trees than H. annosum s.s. In uneven-aged stands, the genets (the area of the same fungal genotype) have been larger than in typical mature even-aged spruce stands (Piri and Valkonen 2013). Moreover, suppression seems to increase the susceptibility of young spruce to Heterobasidion infection (Gibbs 1967). Dense spruce regeneration favours the spread of the disease due to competition stress and more frequent root contacts. In dense regeneration there is a greater number of thinning stumps and the risk of stump infection will increase (Johansson and Pettersson 1996).
The vegetative spread of the fungus can, in theory, be controlled by reducing the number of root contacts between susceptible host trees using mixed stands (or low stand density). Losses caused by the disease in mixed stands are reported to be lower than in pure stands of Scots pine and Norway spruce (e.g. Rennerfelt 1946; Enersvedt and Venn 1979; Lindén and Vollbrect 2002; Piri et al. 1990). However, some studies also report no or only small differences in rot incidence between mixed and pure stands (Piri and Korhonen 2001). In infected stands where H. parviporum is the most frequent decay-causing agent, regeneration with broadleaved trees and Scots pine has been recommended (Korhonen 1978a), although Scots pine is not fully resistant to H. parviporum (Jokinen and Tamminen 1979). In pine stands, the proportion of the mixed species, for example birch, should be more than 50% in order to effectively decrease the incidence of Heterobasidion rot. For control purposes, it would be ideal to grow a pure hardwood (birch) generation in infected Scots pine stands (Piri et al. 1990).
Preventive control of Heterobasidion rot in healthy stands, such as avoidance of summer cuttings, avoidance of mechanical injuries and stump treatment, can be used regardless of the management regime. In already infected stands, many of the most effective control methods, such as prescribed burning, choice of the main tree species and stump removal (Kallio 1965; Stendlid 1987; Cleary et al. 2013) can only be applied in even-aged management. Unfortunately, there are very few options to control Heterobasidion root rot in infected uneven-aged stands (see discussion in Piri and Valkonen 2013).
3.1.2. Gremmeniella abietina
Gremmeniella abietina (Lagerb.) Morelet (anamorph: Brunchorstia pinea (P. Karst.) Höhn.) is the causative agent of the dieback and canker disease on coniferous trees, also called Scleroderris canker. The European race of G. abetina var. abietina is present throughout Europe on Pinus spp., as well as on Picea spp., Larix spp., and other conifers. The disease is epidemic in nature. Severe epidemics can only develop when conditions are unsuitable for the host, and at the same time are suitable for the dispersal and infection by the pathogen.
Many factors in even-aged forestry may increase the damage by G. abietina. The susceptibility of too southern or otherwise incompatible Scots pine provenances has been well demonstrated in Scandinavia. Significant differences in the susceptibility to G. abietina have been found when the seed transfer northwards exceeds 150–200 km or more than 100 d.d. (Dietrichson 1968; Uotila 1985).
If the trees are of local origin, site and stand conditions, together with climatic factors, determine the degree of predisposition to damage of trees and stands. Planting of pines into sites which are too fertile for pine may be one of reasons for the increased susceptibility of even-aged stands. The planted nursery seedlings may also serve as an inoculum source. The disease has been more severe in depressions and on fine textured soils, which formerly might have been Norway spruce stands. The susceptibility of pines is increased in shaded or dense stands (Read 1968; Niemelä et al. 1992; Nevalainen 1999). The disease is more common in fertile sites, at least in mineral soils (Nevalainen 1999). Artificially regenerated stands have been found to be more infected than naturally regenerated ones (Kallio et al. 1985). Since G. abietina has also been found as the primary parasite of young Norway spruce shoots (Barklund and Rowe 1981), in some cases the pathogen may be favoured by conditions in uneven-aged spruce stands (shading, suppression).
3.1.3 Bark beetles
The European spruce bark beetle (Ips typographus L.) is one of the main pests of Norway spruce stands in Fennoscandia and the rest of Europe. The main factors resulting in I. typographus outbreaks are high insect abundance, a sufficient amount of low resistance breeding material, favourable weather conditions and susceptibility of the host trees (Wermelinger 2004; Baier et al. 2007). In Finland, it has been found that fewer than 20 wind-felled spruces in managed forests usually cause no risk to the remaining stand at endemic I. typographus population levels (Eriksson et al. 2007). Severe storms have led to massive outbreaks of the pest, as is well known in Fennoscandia (Christiansen and Bakke 1988; Eidmann 1992; Lindelöw and Schröder 2008).
In uneven-aged or continuous cover stands, the lower density of older trees may make the stands less vulnerable to spruce bark beetle attacks (Klapwijk et al. 2016). Also, the probability of wind-throw is smaller in uneven-aged stands (see below). The initially lower density of bark beetles will reduce the magnitude of a possible outbreak after windthrow (Kärvemo et al. 2014). Moreover, the increased availability of substrate for alternative hosts in uneven-aged stands (small-diameter dying trees and harvesting residuals from frequent selective cuttings) could lead to an increased predator-prey ratio. The increased understorey and structural diversity may also benefit the natural enemies of the bark beetle. The importance of this factor has, however, not been proven.
The six-toothed spruce bark beetle (Pityogenes chalcographus L.) may accompany Ips typographus attacks on older spruce trees. But the species alone also attacks and kills young (10–20-year-old) spruce trees, particularly in connection with drought (Eidmann 1992). The species may be potentially harmful especially in uneven-aged spruce stands.
Pine shoot beetles (Tomicus spp.) are serious pests of Scots pine. The species are considered as secondary pests attacking pines that are weakened by physical stress. The subsequent maturation feeding in young pine shoots causes crown deformation and reduction in growth (Eidmann 1992). These species are efficiently controlled by correctly timed forestry practices: removing beetle breeding material and timing logging so that logging residues have time to dry out before the next spring breeding flight. The suppressed pine trees in any management system, but most probably those in uneven-aged stands, can be expected to be susceptible to pine shoot beetle attack.
3.1.4 Defoliators
The pine sawflies, mainly Neodiprion sertifer Geoffr. and Diprion pini L., are important defoliators in Nordic pine forests. Although N. sertifer has larger-scale outbreaks, the economic impact, considering tree growth and mortality and risk of subsequent damage by bark beetles, is higher in the case of D. pini (Lyytikäinen-Saarenmaa and Tomppo 2002). It is difficult to find differences in sawfly damage risks between forest management regimes.
The two species differ somewhat in host preference. N. sertifer larvae often feed on pole stage stands of Scots pine (Larsson and Tenow 1984), while D. pini favours more mature stands, although the damage is not strictly limited to mature stands. Dominant trees have been the most defoliated in young stands, but not in more mature stands. Potentially this may indicate a higher risk of D. pini damage to taller trees in uneven-aged pine stands, although no concordant effects of stand structure were found by De Somviele et al. (2004).
Large scale outbreaks of N. sertifer occur mostly in vast homogenous Scots pine stands, and there is some evidence of resistance to herbivory through higher rates of predation in mixed stands of pine and birch (Kaitaniemi et al. 2007). However, De Somviele et al. (2004) found no unambiguous proof that tree species composition had a significant impact on the variation in D. pini damage. Compared with outbreaks experienced in mixed stands, outbreaks of D. pini in pure Scots pine stands in France have started earlier, lasted longer and the defoliation has been more severe (Geri 1988).
The black arches or nun moth (Lymantria monacha L.) is considered one of the most destructive defoliator butterfly species in Europe together with the gypsy moth (Lymantria dispar L.) (Bejer 1988). The distribution range covers most of Europe including the most northern part of Fennoscandia, and the species is expanding northwards (Fält-Nardmann et al. 2015). In Finland, it is considered as a southern species and it is not yet considered economically harmful, but the species has increased rapidly in abundance according to the Finnish moth monitoring scheme, Nocturna (Leinonen et al. 2016). The nun moth can feed on a wide range of hosts and develops to maturity on broadleaved trees as well as on coniferous hosts. Outbreaks seem to start in old stands of either Norway spruce or Scots pine. Although large outbreaks often occur in large pure spruce stands, there seems to be no relation between damage risk and compositional diversity or size of the stand (Bejer 1988), and thus, no relation to management regimes.
3.1.5 The large pine weevil
The large pine weevil (Hylobius abietis L.) is by far the most serious cause of damage to both Norway spruce and Scots pine seedlings in southern Finland, Sweden and Norway. The risk of seedling damage by the weevil in unprotected seedlings can be more than 75% after planting (Nilsson et al. 2010; Mattson 2016). Small seedlings are more sensitive.
The species reproduces in stumps and logging residues and the adults of the species attack newly planted seedlings. Thus, the main factor limiting the abundance of the pine weevil is the availability of fresh conifer stumps as breeding material. It takes from two to five years for the larvae to develop into adults (von Sydow 1997; Långström and Day 2004).
In even-aged forestry the risk of pine weevil damage can be minimized by several measures, for example, choice of seedling type, timing of planting, site preparation, retention of shelter trees, and direct seedling protection by insecticides or various seedling covers (von Sydow 1997; Nordlander et al. 2011). The risk of seedling damage by the weevil is higher in unprepared soil or if prepared soil is covered by humus or a mixture of humus and mineral soil. Mounding has significantly decreased pine-weevil damage of planted Norway spruce seedlings (Petersson et al. 2005; Heiskanen and Viiri 2005; Luoranen and Viiri 2012). In uneven-aged forestry the smaller number and increased shading of stumps can be expected to decrease the number of weevils and increase the development time of the weevil larvae, making them more vulnerable to parasites or predators (Inward et al. 2012). The soil preparation method also affects the survival of outplanted seedlings. For instance, ditch or spot mounding should be used on frost-heave-susceptible forest soils to promote plantation establishment of Norway spruce (Heiskanen et al. 2013).
3.1.6 Moose
Moose (Alces alces L.) cause large economic losses to forestry in Fennoscandia, especially due to their winter browsing of Scots pine sapling stands (Helle et al. 1987; Lavsund 1987; Bergqvist et al. 2014). There are no reports comparing moose damage risks in even- and uneven-aged stands in boreal forests. Even-aged forestry has aimed at an economically optimal age-class distribution of the forests with a high proportion of young forest, which has also favoured the moose (Markgren 1974; Lavsund et al. 2003). The structural complexity of uneven-aged stands or the effect of small clearings (patch harvesting) has not been studied regarding moose damage risk.
However, in even-aged forestry, artificially regenerated stands have had more damage than naturally regenerated stands, although the results concerning this point are very scarce. Also soil scarification, such as ploughing or mounding, has increased the probability of browsing (Jalkanen et al. 2005; Nikula et al. 2008; Nevalainen et al. 2016).
Studies of tree species composition or the density of seedling stands have produced contradictory results regarding whether these factors increase or decrease moose damage (Heikkilä and Mikkonen 1992; Heikkilä and Härkönen 1996; Ball and Dahlgren 2002). Increased proportions of broadleaved species, especially aspen, but also overtopping birches, have increased the risk of moose damage, as compared to pure Scots pine sapling stands in many studies (Nikula et al. 2008; Härkönen et al. 2008; Bergqvist et al. 2014; Nevalainen et al. 2016). On the other hand, clearing of broadleaves may increase the browsing pressure on, and the severity of damage to, the remaining conifers (Heikkilä and Mikkonen 1992; Lyly and Saksa 1992).
3.1.7 Wind damage
Many factors, besides wind speed, contribute to the physical stability of trees. These include the effects of topography, soil type and canopy characteristics (tree species, diameter, height and stand density) (Dhôte 2005; Zeng et al. 2007). The level of wind damage risk thus also depends greatly on forest management. Damage is most probable in stands adjacent to newly clearcut areas or in stands that have recently been heavily thinned (Valinger and Pettersson 1996; Gardiner et al. 1997; Peltola et al. 1999). Small clearings can reduce the wind speeds. On the other hand, the risks at stand or landscape levels may be different (see Zeng et al. 2007 and the references therein).
Much of the research on wind damage is based on mechanistic models (Blennow and Sallnäs 2004; Peltola et al. 2013), and there are but a few direct comparisons between different forest management systems which could be applied to Nordic conditions. Even-aged spruce stands have been shown to be more susceptible to windthrow than uneven-aged stands (Shorohova et al. 2008). The uneven-aged structure of the investigated silver fir (Abies alba Mill.) and Norway spruce forests in Switzerland may be one reason for the low levels of storm damage (Hanewinkel et al. 2014).
Recently Pukkala et al. (2016) reported that the highest-risk cutting was shelterwood cutting, followed by even-aged silviculture characterized by repeated low thinnings. The probability of wind damage (wind throw) was lowest in selective high thinnings of uneven-aged stands, and in dimension cuttings. The damage rates were higher in typical mature even-aged structured stands compared to uneven-aged stands.
A strong linear relationship has been found between thinning intensity and the frequency of damage caused by storms and snow in Norway spruce stands. Wind damage was best predicted by stand basal area left after thinning and stand age (Valinger and Pettersson 1996).
3.1.8 Mechanical injuries
Thinning and logging operations in any management system should be conducted so that mechanical damage caused to the residual stand is minimized. The classic study of Isomäki and Kallio (1974) showed that mechanical injuries in (even-aged) Norway spruce stands are an important source of stem decay due to, for example, Heterobasidion rot and also cause growth reductions.
The long-term future of an uneven-aged Norway spruce stand depends on emergence, survival, ingrowth and growth of small trees. The studies concerning the proportion of mechanically damaged trees in uneven-aged stands show somewhat variable results, depending on, for example, whether the damage was studied on mature trees or advanced growth, seedlings or saplings, and which symptoms were recorded as damage. In general, the damage risk is high especially in small lower canopy trees, and is largely dependent on the density of the stand, harvesting intensity, distance from the nearest skid row, and the operating system used. Logging has caused death or injury of up to 30–40% of small spruces. The average annual mortality rates of small spruce seedlings have been in the range of 2–7%. However, it is often difficult to make a distinction between the disappearance of small seedlings due to logging or due to natural mortality (Fjeld and Granhus 1998; Granhus and Fjeld 2001; Nilson and Lundqvist 2001; Surakka et al. 2011; Modig et al. 2012; Eerikäinen et al. 2014).
Mechanized harvesting operations can be more challenging in uneven-aged forestry. In selection cuttings of the uneven-aged stands it can be difficult to achieve the lower damage levels typically found in even-aged forestry. If a significant percentage of these trees are damaged in harvesting, repeated cuttings every 15–20 years are not possible (Siren et al. 2015; Surakka et al. 2011).
3.1.9 Other causes of damage
Voles, such the short-tailed vole (Microtus agrestis L.) and the bank vole (Myodes glareolus Schreber, syn. Clethrionomys glareolus Schreber) cause damage to seedlings by gnawing bark and buds. Vole damage may also predispose the seedlings to wound pathogens (e.g. Henttonen et al. 1994). According to a nationwide survey in Finland, roughly 80% of all vole damage was inflicted on Norway spruce. The occurrence of damage during the winter is positively related to vole abundance in the previous autumn (Huitu et al. 2009), making weed control a necessity in the highest-risk sites. Voles have shown higher preference for planted than naturally regenerated Norway spruce seedlings (Virjamo et al. 2013), which indicates a higher risk in even-aged forestry.
Species of Armillaria (A. borealis Marxm. & Korhonen, A. cepistipes Velen., A. solidipes Peck [syn. A. ostoyae Romagnesi]) are common in Fennoscandian forests. Infection by these fungi is through soil-growing rhizomorphs and/or by roots contacting diseased trees or stumps. A. borealis and A. cepistipes are common in declining and dead trees. Armillaria species are also common in Norway spruce trees with butt rot. In southern Finland, A. solidipes has killed small Scots pine seedlings (Korhonen 1978b; Hallaksela 1984; Guillaumin et al. 1993; Keča and Solheim 2011).
Decreased mortality of susceptible conifers by Armillaria root rot has been observed in mixed conifer and broadleaved stands in Canada in long-term experiments (Morrison et al. 2014). On the other hand, stumps of deciduous trees may serve as inoculum sources, and seedlings or weakened trees may die (Rishbeth 1978). Thus, stump removal is also effective in reducing disease incidence by Armillaria species (Cleary et al. 2013).
Planting in even-aged forestry has created homogenous seedling stands, which may be susceptible to many diseases, such as grey needle cast (Lohdodermella sulcigena [Rostr.] Höhn.) or pine twisting rust (Melampsora pinitorqua [Braun] Rostr.) Moreover, Scots pine has sometimes been planted on unsuitable sites, for instance on sites that are too fertile, which makes the trees more susceptible to many diseases (Jalkanen 1985, 1986; Jalkanen and Kurkela 1984).
Fertilization is commonly done in intensive (even-aged) forest management. Regarding susceptibility to most diseases and disorders, balanced nutrient ratios are apparently more important than the level of any single nutrient (Jokinen 1983). Excessive nitrogen fertilization has caused needle damage to Scots pine in Lapland (Jalkanen 1990).
Growth disturbances have been observed in peatland stands, particularly in trees growing on afforested former agricultural peat soils (see Kolari 1983 and the articles cited therein). Typically, the trees have high foliar nitrogen and phosphorus concentrations and low foliar boron, copper and zinc concentrations. These cases can be attributed to practices in even-aged forestry. Growth disturbances have also been reported in Norway spruce trees on fertile soils with a slash-and-burn or pasturing history in many parts of eastern Finland. The symptoms are typical of boron deficiency (Rikala 2004).
3.2 Main results of the expert survey
The scaled damage risk (all causes of damage and operational chains combined) were higher in even-aged management, in both Norway spruce and Scots pine. Scots pine was estimated to have more damage than Norway spruce in both management regimes (Fig. 1). The highest risks in Scots pine were caused by moose (even-aged chains) and harvesting damage (uneven-aged chains). In Norway spruce, root rot caused the highest risks both in even- and in uneven-aged chains (Fig. 2). The variation in the scaled damage risks was large, however.
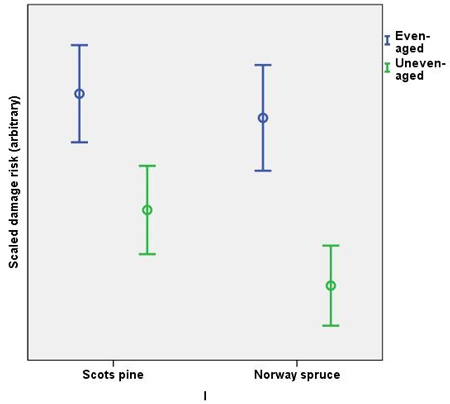
Fig. 1. Scaled damage risks in the management regimes by main tree species. All damage causes combined. The error bars show 95% confidence intervals, but the actual scale of the arbitrary y-axis is not shown.
The scaled damage risks by causal agent (all chains within a regime combined) were usually estimated to be higher in even-aged management. In Scots pine 12 of the 21 estimated damage risks were higher in even-aged management, and risks were higher regarding four causes of damage in uneven-aged management (Table 4). No differences were found in pine shoot beetle or large pine weevil damage, for instance. In Norway spruce, none of the 16 estimated damage risks were higher in uneven-aged management, while risks were estimated to be higher for 11 causes of damage in even-aged management. Root rot damage was estimated to be much higher in Scots pine in uneven-aged than in even-aged management, but this difference was not observed in Norway spruce (Fig. 2, Table 4).
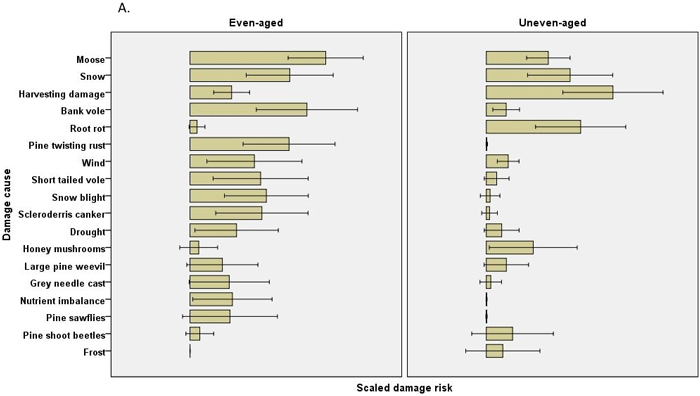
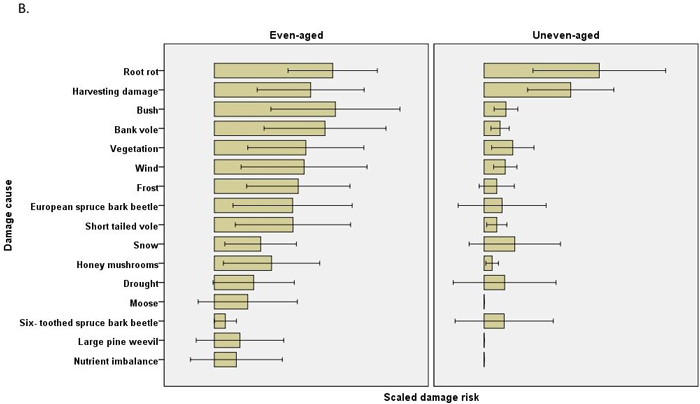
Fig. 2. The risks (scaled damage risks) by damage causes by management regimes and by main tree species: A) Scots pine B) Norway spruce. The error bars show 95% confidence intervals, but the actual scale of the arbitrary x-axis is not shown.
When the individual chains were examined, especially high values of the scaled damage risk (all causes combined) were found in the even-aged pine chains 17–19, which represented the clearcut method and soil harrowing in North Finland (Fig. 3). The clearcut chain at mesic sites in South Finland (chain 4) had high risk values. In Norway spruce chains the highest risks occurred in the shelterwood, strip felling and clearcut chains (chains 9, 7 and 5, respectively, the latter representing herb-rich sites without soil preparation). In uneven-aged spruce chains, the ones with patch (chain 28) and selection felling (chain 29) had the highest risk values (Fig 3). (See Table 1 for a full description of the chains.)
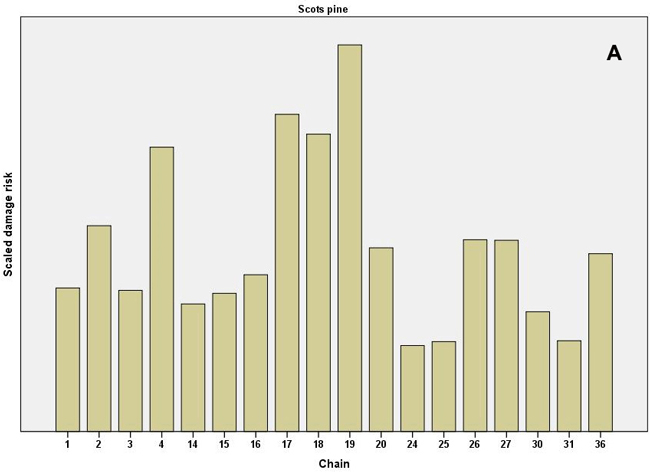
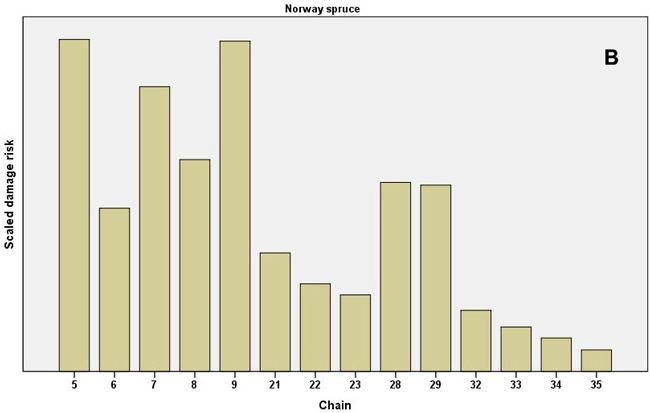
Fig. 3. Scaled damage risks by the operational chains used in the expert survey. All damage causes combined. A) Scots pine as the main tree species and B) Norway spruce as the main tree species. For description of the chains, see Table 1. Chains 24–36 present uneven-aged chains, as shown.
The damage risks for the individual causes of damage differed between chains. For example, the Norway spruce even-aged, clearcut, herb-rich chain (chain 5) demonstrated high values for frost, snow and vole damage and other regeneration problems, while the shelterwood chain (chain 9) was estimated to have higher risks of harvesting damage and root rot (Table 2, Fig. 3). The uneven-aged Norway spruce chains with patch and selection fellings with natural regeneration or regeneration utilizing undergrowth (chains 28 and 29, respectively) also had high risks for root rot and harvesting damage. The high root rot risks in uneven-aged Scots pine were found in the chains for South Finland, with patch or selection felling, natural regeneration or utilization of undergrowth (chains 25–27). In the chain for clearcut, even-aged Scots pine in mesic or subxeric soil in North Finland (chain 19) the risks were estimated to be high for voles, moose and pine twisting rust (Table 2).
| Table 2a. Risk of damage (scaled damage risk) by the causal agent and operational chain, in chains with Scots pine as the main tree species. Risk classes: L = low risk M = medium risk, H = high risk. The risk classes are based on percentiles of the scaled damage risks of the specific cause in all the chains: 51–70 percentiles present a medium risk and percentiles over 70 a high risk. For a description of the chains, please see Table 1. | |||||||||||||||||
| Damage cause | Chain number (even-aged management) | Chain number (uneven-aged management) | |||||||||||||||
| 1 | 2 | 3 | 4 | 14 | 15 | 16 | 17 | 18 | 19 | 24 | 25 | 26 | 27 | 30 | 31 | 36 | |
| Drought | H | H | H | L | M | L | L | M | L | L | M | L | M | M | L | L | L |
| Frost | L | L | L | L | L | L | L | L | L | L | L | H | L | L | L | L | L |
| Nutrient imbalance | L | H | L | H | L | L | L | M | L | H | L | L | L | L | L | L | L |
| Snow | M | M | H | H | H | H | H | H | H | H | M | M | H | H | H | H | H |
| Wind | L | L | H | L | L | H | H | M | L | H | M | L | M | M | M | L | M |
| Large pine weevil | M | L | L | L | L | L | M | L | H | M | M | M | L | L | L | L | M |
| Pine sawflies | H | H | H | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| Pine shoot beetle | M | L | L | L | L | L | L | L | L | M | L | L | L | H | L | L | |
| Harvesting damage | M | M | H | M | L | M | M | M | M | H | M | M | H | H | H | M | H |
| Bush | M | H | M | H | L | L | L | M | L | M | L | M | L | M | L | M | L |
| Vegetation | M | M | H | H | L | L | L | M | H | M | L | M | L | M | L | M | H |
| Bank vole | H | H | M | H | H | H | H | H | H | H | M | M | L | L | M | M | M |
| Short tailed vole | M | M | L | M | M | M | M | H | H | H | L | L | L | L | L | M | M |
| Moose | M | H | H | H | M | M | H | H | H | H | M | H | H | M | H | H | L |
| Grey needle cast | L | M | L | M | L | L | L | H | L | H | L | L | L | L | L | M | L |
| Honey mushrooms | L | M | L | L | L | L | L | L | L | L | M | L | H | H | L | M | M |
| Pine twisting rust | M | H | L | H | L | L | M | H | H | H | L | L | L | L | L | L | L |
| Resin-top disease | L | L | L | M | L | L | L | H | M | H | L | L | M | M | L | L | L |
| Root rot | L | L | M | M | L | L | L | L | L | L | M | H | H | H | L | L | H |
| Scleroderris canker | H | H | L | H | L | H | M | H | H | M | L | L | L | L | M | L | L |
| Snow blight | L | M | L | H | H | H | H | H | M | L | L | L | L | M | H | L | |
| Table 2b. Risk of damage (scaled damage risk) by the causal agent and operational chain, in chains with Norway spruce as the main tree species. Risk classes: L = low risk M = medium risk, H = high risk. The risk classes are based on percentiles of the scaled damage risks of the specific cause in all the chains: 51–70 percentiles present a medium risk and percentiles over 70 a high risk. For a description of the chains, please see Table 1. | ||||||||||||||
| Damage cause | Chain number (even-aged management) | Chain number (uneven-aged management) | ||||||||||||
| 5 | 6 | 7 | 8 | 9 | 21 | 22 | 23 | 28 | 29 | 32 | 33 | 34 | 35 | |
| Drought | L | M | M | L | L | H | L | L | L | H | L | L | L | L |
| Frost | H | M | H | H | M | M | M | M | M | L | M | L | L | L |
| Nutrient imbalance | L | L | L | L | L | H | L | L | L | L | L | L | L | L |
| Snow | H | L | H | L | M | M | M | M | M | M | H | L | L | L |
| Wind | L | L | H | H | H | L | H | H | L | M | M | M | M | M |
| European spruce bark beetle | M | M | H | H | L | L | L | L | M | L | L | L | L | L |
| Large pine weevil | H | M | L | L | L | L | L | L | L | L | L | L | L | L |
| Six toothed spruce bark beetle | L | M | M | M | L | L | L | L | M | L | L | L | L | L |
| Harvesting damage | M | M | H | H | H | L | M | M | H | H | L | M | M | L |
| Bush | H | L | H | H | H | L | M | L | M | M | M | M | L | L |
| Vegetation | H | L | H | M | H | L | M | L | M | M | M | M | L | L |
| Bank vole | H | H | M | M | M | H | M | M | M | L | M | M | L | L |
| Short tailed vole | H | H | M | M | M | H | M | M | M | M | L | L | L | L |
| Moose | H | H | L | L | L | L | L | L | L | L | L | L | L | L |
| Honey mushrooms | M | M | M | M | H | M | L | L | L | L | L | L | L | L |
| Root rot | H | H | H | H | H | L | L | L | H | H | L | L | L | L |
Various combinations of causes of damage, operational methods, cutting methods, site factors, etc. lead to very different risk values. For Norway spruce, for instance, the highest risk values (scaled damage risk value of 2144) were found in South Finland, when the damage cause was root rot or harvesting damage and the cutting method was patch harvesting, selective or shelterwood cutting (Fig. 4). The lowest risk values (scaled damage risk value of 29), in turn, were found in mesic sites in South Finland, when the cause of damage was bush, vegetation, frost or bank vole. The risks were also relatively small in North Finland. The highest-risk operations (Question B) according to the experts’ opinions (the points or the votes given) were clearcutting, selection cutting, thinning operations, strip felling, and planting (Table 3).
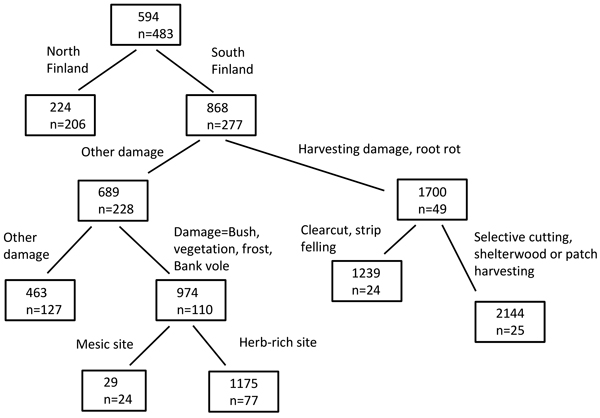
Fig. 4. Example of a regression tree, computed with rpart package in R. The scaled damage risk was used as the response variable, and combinations of operational methods, site factors, damage causes and cutting methods were used as classifiers. The numbers in the box show the risk value (scaled damage risk) and n indicates the number of risk assessments (by all experts and for the appropriate damage causes and operational chains).
| Table 3. The 10 most risky operations (question B), based on the votes given by the experts). EA = even-aged, UEA = uneven-aged management. Votes means the votes given by all experts for all damage causes. | |||
| Regime | Tree Species | Operation | Votes |
| EA | Scots pine | Clearcutting | 67 |
| UEA | Scots pine | Selection cutting | 66 |
| EA | Norway spruce | Thinning | 62 |
| EA | Scots pine | Thinning | 54 |
| EA | Norway spruce | Strip felling | 46 |
| EA | Scots pine | Planting | 41 |
| UEA | Norway spruce | Patch harvesting | 39 |
| EA | Norway spruce | No soil preparation | 36 |
| UEA | Norway spruce | Selection cutting | 33 |
| EA | Norway spruce | Clearcutting | 33 |
3.3 Comparison of the literature review and the expert survey
The findings in the literature review and the results from the expert survey support each other quite well in general. However, some discrepancies were found. Surprisingly, root rot damage risk in stands with Scots pine as the main tree species (all chains combined) was estimated to be much higher in uneven-aged management chains, while this difference was not observed in Norway spruce stands. However, there were big differences between the chains even within the same regime (Table 4).
| Table 4. Comparison of damage risks between even-aged (EA) and uneven-aged (UEA) management regimes according to the literature review and the expert survey. Blank cells in the literature review columns indicate no data. The comparisons in the expert survey are based on the scaled damage risk. The risk values over two times higher or lower depicted a higher or lower risk, respectively. Values five times higher or lower depicted a much higher or lower risk. ’>>’ denotes a much higher risk and ‘<<’ a much lower risk. The most obvious discrepancies between the literature and the expert survey are underlined. | |||
| Main tree species | Damage cause | Comparison of damage risks in EA and UEA in the literature review | Comparison in the expert survey |
| Sots pine | Drought | Seedling damage may be higher in EA | EA > UEA |
| Frost | Seedling damage may be higher in EA | EA << UEA | |
| Nutrient imbalance | Increased risk in former peatlands in EA | EA >> UEA | |
| Snow | EA = UEA | ||
| Wind | Higher risk in EA | EA > UEA | |
| Large pine weevil | Higher risk in EA | EA = UEA | |
| Pine sawflies | No unambiguous data | EA >> UEA | |
| Pine shoot beetles | No clear differences | EA = UEA | |
| Harvesting damage | Usually low risk in EA and UEA | EA < UEA | |
| Bush | EA > UEA | ||
| Vegetation | EA = UEA | ||
| Bank vole | EA >> UEA | ||
| Short tailed vole | Planted seedlings: higher risk in EA | EA >> UEA | |
| Moose | Higher damage in EA seedling stands | EA > UEA | |
| Grey needle cast | Higher risk in EA in too fertile soils | EA >> UEA | |
| Honey mushrooms | No clear differences | EA < UEA | |
| Pine twisting rust | EA >> UEA | ||
| Resin- top disease | EA > UEA | ||
| Root rot | High risk in EA and UEA | EA << UEA | |
| Scleroderris canker | Higher risk in EA | EA >> UEA | |
| Snow blight | EA >> UEA | ||
| Norway spruce | Drought | Seedling damage may be higher in EA | EA = UEA |
| Frost | Seedling damage may be higher in EA | EA >> UEA | |
| Nutrient imbalance | Higher risk in EA | EA >> UEA | |
| Snow | EA = UEA | ||
| Wind | Higher risk in EA | EA > UEA | |
| European spruce bark beetle | Higher risk in EA | EA > UEA | |
| Large pine weevil | Higher risk in EA | EA >> UEA | |
| 6- toothed spruce bark beetle | No clear differences | EA = UEA | |
| Harvesting damage | Repeated thinnings: high risk in UEA | EA = UEA | |
| Bush | EA >> UEA | ||
| Vegetation | EA > UEA | ||
| Bank vole | EA >> UEA | ||
| Short tailed vole | Planted seedlings: higher risk in EA | EA >> UEA | |
| Moose | Small risk in EA and UEA | EA >> UEA | |
| Honey mushrooms | No unambiguous data | EA >> UEA | |
| Root rot | High risk in EA and UEA | EA = UEA | |
4 Discussion
Based on both the literature review and the expert survey, the damage risks are usually higher in even-aged forestry. This is largely due to the many practices within even-aged forestry that favour various types of damage. These practices include, for example, the planting of trees on unsuitable sites, the use of nursery seedlings, seed transfer, year-round harvesting, mechanized harvesting and fertilization. Many of these effects are cited in the review section of this article.
Kallio and Laine (1982) stated that the operations reducing forest hygiene in intensified (even-aged) forestry management include year-round and mechanized harvesting, fertilization and use of nursery seedlings. Based on studies from model pathosystems, tree disease epidemics are often facilitated by forest management practices (Weissenberg 1987). Forestry practices are known to strongly influence the susceptibility of stands to insect pests and other biotic and abiotic hazards. For instance, the pine forests in the Karelian Isthmus in Russia, which receive almost no management, suffered more from damage by pine shoot beetles (Tomicus spp.) while Gremeniella abietina was more common in managed Finnish pine forests (Lumme et al. 1997).
However, there are some important exceptions: the damage risks may be higher in uneven-aged stands in some cases. In Heterobasidion-infected Norway spruce stands especially, the utilization of undergrowth or natural regeneration should be avoided. In selection cuttings of uneven-aged stands it can be difficult to achieve the lower damage levels typically found in even-aged forestry, which may make repeated thinnings unfeasible. Development both in working methods and in machinery is important in order to reduce sapling damage in selection cuttings (Surakka et al. 2011).
The data obtained from the survey was quite heterogeneous. This can be due to the rather small number of experts surveyed, and due to their varying specializations in damaging agents. The conversion of the survey results into the variable “scaled damage risk” helped to commensurate the results somewhat, but on the other hand produced an arbitrary scale. Many different scaling and normalization methods were tested, but the main results do not change much, regardless of the scale used. Although the survey results in question A were in this way generalized to minimize the effect of single respondents, a high risk in a few chains may have affected the result in the summarized data. For instance, the high risks of root rot in uneven-aged Scots pine were found in only two chains, in which the cutting method was selective cutting. The risk of root rot in Norway spruce stands was estimated to be similar in both management regimes in the survey. This, in turn, was due to the high risks in five even-aged chains, but high risks in (only) two uneven-aged management chains. Similarly, higher frost risk in uneven-aged Scots pine in the survey (Table 2) can be explained by an estimation of high risk in one chain (chain 25), but low risks in all the other chains (Table 3).
It is often assumed that forest monocultures (monospecific stands) are prone to pest outbreaks and disease epidemics, and in turn, mixed stands are considered less susceptible, at least to widespread pest and disease damage. Many studies suggest that foliar and root pathogen abundance and disease severity decrease with increasing tree species diversity (Jactel et al. 2005; Pautasso et al. 2005; and Björkman et al. 2011). Much of the literature on various aspects of diversity and damage, however, covers temperate and tropical forests, and comparisons between plantation and natural forests cannot be adapted to Nordic boreal forests. According to Koricheva et al. (2006) empirical evidence supporting the lesser susceptibility of mixed forest stands to herbivores and pathogens is largely circumstantial, and quite controversial, although beneficial effects of tree-species diversity on stand vulnerability can be observed in some cases.
The interaction with other stress factors must be considered. For instance, root rot makes the trees prone to other damage factors such as wind (Oliva et al. 2008). Also, drought conditions predispose conifers to Heterobasidion infection (Lindberg and Johansson 1992) and to spruce bark beetle (Marini et al. 2013). Moreover, decayed stands are more vulnerable to storm damage, and in turn to bark beetle attacks.
The warming climate will also affect damage risks. Lindner et al. (2010) state that the potential risk of high impact responses to different disturbances is increasing under climate change. For instance, storm damage seems to have increased both in frequency and in magnitude and may become even more frequent in the future (Schelhaas et al. 2003). Concerning the European spruce bark beetle the prolonged and warmer summers enable earlier swarming in the spring, faster oviposition, development and maturation which result in a shorter generation cycle. Two generations have been observed in southern Sweden and the same has also occurred in Finland (Lindelöw and Schröder 2008; Pouttu and Annila 2010).
Thinning operations and timber extraction during longer snow-free and ground-frost-free periods provide fresh stump surfaces and injured roots/trunks for the establishment of Heterobasidion spp. Seven of the eight factors attributed to climate change also increase the risk of root rot (Müller et al. 2012). It is expected that the development time of larvae of the large pine weevil (Hylobius abietis) may shorten and a portion of the adults may emerge in autumn instead of overwintering and emerging in the following spring, resulting in a higher seedling mortality in the future (Björkman et al. 2011). The potentially damaging nun moth (Lymantria monacha) will expand its distribution range northwards due to climate change and thus also its outbreak capabilities will increase significantly in northern locations (Vanhanen et al. 2007). It has been predicted that increased winter temperatures may increase the frequency of outbreaks of Neodidrion sertifer in Finland, especially in the north (Virtanen et al. 1996). The factors affecting many diseases and pests are complex, however. For instance, a warmer climate may be harmful for the European pine sawfly (Kollberg et al. 2013).
The control of forest diseases and pests should be preventive, since direct control methods are most often impossible. Regardless of the management regime, it is important to take care of forest hygiene in order to avoid subsequent damage to the growing stock. By adopting suitable silvicultural operations damage problems can be minimized in future forests, without compromising other forest management objectives. Due to the complexity of the interactions involved, close collaboration is required between researchers from different disciplines (Jactel et al. 2009; Björkman et al. 2011).
Adaptive forest management practices will be necessary to increase the resistance and resilience of forests and to mitigate the effects of climate change, regardless of the chosen management strategy. These should include changes in thinning regimes, reduction of rotation length, and using the most suitable tree species (Subramanian et al. 2016).
Acknowledgements
The author wishes to thank the following colleagues for their devoted participation in the damage survey: Otso Huitu, Risto Jalkanen, Juho Matala, Jari Miina, Tuula Piri, Marja Poteri and Sauli Valkonen (all from the Natural Resources Institute Finland).
References
Äijälä O., Koistinen A., Sved J., Vanhatalo K., Väisänen P. (2014). Metsänhoidon suositukset. [Recommendations for silviculture]. Metsätalouden kehittämiskeskus Tapion julkaisuja. 181 p. [In Finnish].
Baier P., Pennerstorfer J.,Schopf A. (2007). PHENIPS – a comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. Forest Ecology and Management 249(3): 171–186. https://doi.org/10.1016/j.foreco.2007.05.020.
Ball J.P., Dahlgren J. (2002). Browsing damage on pine (P. sylvestris and P. contorta) by a migrating moose (Alces alces) population in winter: relation to habitat composition and road barriers. Scandinavian Journal of Forest Research 17(5): 427–435. https://doi.org/10.1080/028275802320435441.
Barklund P., Rowe J. (1981). Gremmeniella abietina (Scleroderris lagerbergii), a primary parasite in a Norway spruce die-back. European Journal of Forest Pathology 11(1–2): 97–108. https://doi.org/10.1111/j.1439-0329.1981.tb00075.x.
Bejer B. (1988). The nun moth on European spruce forest. In: Berryman A. (ed.). Dynamics of forest insect populations. Plenum Press, New York. p. 211–231. https://doi.org/10.1007/978-1-4899-0789-9_11.
Bendz-Hellgren M., Stenlid J. (1998). Effects of clear-cutting, thinning, and wood moisture content on the susceptibility of Norway spruce stumps to Heterobasidion annosum. Canadian Journal of Forest Research 28(5): 759–765. https://doi.org/10.1139/x98-043 .
Bendz-Hellgren M., Brandtberg P., Johansson M., Swedjemark G.,Stenlid J. (1999). Growth rate of Heterobasidion annosum in Picea abies established on forest land and arable land. Scandinavian Journal of Forest Research 14: 402–407. https://doi.org/10.1080/02827589950154104 .
Bergqvist G., Bergström R., Wallgren M. (2014). Recent browsing damage by moose on Scots pine, birch and aspen in young commercial forests – effects of forage availability, moose population density and site productivity. Silva Fennica 48(1) article 1077. https://doi.org/10.14214/sf.1077.
Björkman C., Bylund H., Klapwijk M.J., Kollberg I., Schroeder M. (2011). Insect pests in future forests: more severe problems? Forests 2(2): 474–485. https://doi.org/10.3390/f2020474 .
Björkman C., Bylund H., Nilsson U., Nordlander G., Schroeder M. (2015). Effects of new forest management on insect damage risk in a changing climate. In: CABI climate change series. CAB International. p. 248–266. https://doi.org/10.1079/9781780643786.0248.
Blennow K., Sallnäs O. (2004). WINDA- a system of models for assessing the probability of wind damage to forest stands within a landscape. Ecological Modelling 175(1): 87–99. https://doi.org/10.1016/j.ecolmodel.2003.10.009.
Christiansen E., Bakke A. (1988). The spruce bark beetle of Eurasia. In: Berryman A. (ed.). Dynamics of forest insect populations. Plenum Press, New York. p. 479–503. https://doi.org/10.1007/978-1-4899-0789-9_23.
Cleary M.R., Arhipova N., Morrison D.J., Thomsen I.M., Sturrock R.N., Vasaitis R., Gaitnieks T., Stenlid J. (2013). Stump removal to control root disease in Canada and Scandinavia: a synthesis of results from long-term trials. Forest Ecology and Management 290: 5–14. https://doi.org/10.1016/j.foreco.2012.05.040.
De Somviele B., Lyytikäinen-Saarenmaa P., Niemelä P. (2004). Sawfly (Hym., Diprionidae) outbreaks on Scots pine: effect of stand structure, site quality and relative tree position on defoliation intensity. Forest Ecology and Management 194(1–3): 305–317. https://doi.org/10.1016/j.foreco.2004.02.023.
Dhôte J.E. (2005). Implication of forest diversity in resistance to strong winds. In: Scherer-Lorenzen M., Körner C., Schulze E.D., (eds.). Forest diversity and function temperate and boreal systems. Springer. p. 291–307. https://doi.org/10.1007/3-540-26599-6_14.
Diaci J., Kerr G., O’hara K. (2011). Twenty-first century forestry: integrating ecologically based, uneven-aged silviculture with increased demands on forests. Forestry 84(5): 463–465. https://doi.org/10.1093/forestry/cpr053 .
Dietrichson J. (1968). Provenance and resistance to Scleroderris lagerbergii Gremmen (Crumenula abietina Lagerb.). The international Scots pine provenance experiment of 1938 at Matrand. Meddelelser fra Det Norske Skogforsøksvesen 25: 398–410.
Eerikäinen K., Valkonen S., Saksa T. (2014). Ingrowth, survival and height growth of small trees in uneven-aged Picea abies stands in southern Finland. Forest Ecosystems 1:5. 10 p. https://doi.org/10.1186/2197-5620-1-5.
Eidmann H. (1992). Impact of bark beetles on forests and forestry in Sweden. Journal of Applied Entomology 114(1–5): 193–200. https://doi.org/10.1111/j.1439-0418.1992.tb01114.x.
Enerstvedt L.I., Venn K. (1979). Decay in mature Norway spruce stands. A study on clear-cuttings in Ovre Eiker. Meddelelser fra Norsk Institutt for Skogforskning 35(4): 241–264.
Eriksson M., Neuvonen S., Roininen H. (2007). Retention of wind-felled trees and the risk of consequential tree mortality by the European spruce bark beetle Ips typographus in Finland. Scandinavian Journal of Forest Research 22(6): 516–523. https://doi.org/10.1080/02827580701800466.
Fält-Nardmann J., Leinonen R., Ruohomäki K., Saikkonen K., Tikkanen O., Neuvonen S. (2015). The recent northward expansion of Lymantria monacha in Finland. IUFRO conference Population dynamics and integrated control of forest defoliating and other insects, September 28 – October 2, Sopot, Poland, Book of abstracts. p. 21.
FAO (2001). Protecting plantations from pests and diseases. Report based on the work of W.M. Ciesla. Forest Plantation Thematic Papers, Working Paper 10. Forest Resources Development Service, Forest Resources Division. FAO, Rome.
Fjeld D., Granhus A. (1998). Injuries after selection harvesting in multi-storied spruce stands – the influence of operating systems and harvest intensity. Journal of Forest Engineering 9: 33–40. .
Gardiner B.A., Stacey G.R., Belcher R.E., Wood C.J. (1997). Field and wind tunnel assessments of the implications of respacing and thinning for tree stability. Forestry 70(3): 233–252. https://doi.org/10.1093/forestry/70.3.233.
Geri C. (1988). The pine sawfly in central France. In: Berryman A. (ed.). Dynamics of forest insect populations: patterns, causes and implications. Plenum Press, New York. p. 377–405. ISBN 978-1-4899-0789-9.
Gibbs J.N. (1967). The role of host vigour in the susceptibility of pines to Fomes annosus. Annals of Botany 31: 803–815.
Graber D. (1994). Die Fichtenkernfäule in der Nordschweiz: Schadenausmass, ökologische Zusammenhänge und waldbauliche Massnahmen. Schweizerische. Zeitschrift für Forstwesen 145: 905–925.
Granhus A., Fjeld D. (2001). Spatial distribution of injuries to Norway spruce advance growth after selection harvesting. Canadian Journal of Forest Research 31(11): 1903–1913. https://doi.org/10.1139/cjfr-31-11-1903.
Guillaumin J.J., Mohammed C., Intin M., Anselmi N., Courtecuisse R., Gregory S.C., Holdenrieder O., Rishbet J., Lung B., Marxmuller H., Morrison D., Termorshuizen A.J., van Dam B. (1993). Geographical distribution and ecology of the Armillaria species in Western Europe. European Journal of Forest Pathology 23(6–7): 321–341. https://doi.org/10.1111/j.1439-0329.1993.tb00814.x.
Gunulf A., Wang L., Englund J., Rönnberg J. (2013). Secondary spread of Heterobasidion parviporum from small Norway spruce stumps to adjacent trees. Forest Ecology and Management 287: 1–8. https://doi.org/10.1016/j.foreco.2012.09.011.
Hallaksela A. (1984). Causal agents of butt-rot in Norway spruce in southern Finland. Silva Fennica 18(3): 237–243. https://doi.org/10.14214/sf.a15395.
Hanewinkel M., Kuhn T., Bugmann H., Lanz A., Brang P. (2014). Vulnerability of uneven-aged forests to storm damage. Forestry 87(4): 525–534. https://doi.org/10.1093/forestry/cpu008.
Härkonen S., Miina J., Saksa T. (2008). Effect of cleaning methods in mixed pine-deciduous stands on moose damage to Scots pines in southern Finland. Scandinavian Journal of Forest Research 23(6): 491–500. https://doi.org/10.1080/02827580802491371.
Heikkilä R., Härkönen S. (1996). Moose browsing in young Scots pine stands in relation to forest management. Forest Ecology and Management 88(1–2): 179–186. https://doi.org/10.1016/s0378-1127(96)03823-6.
Heikkilä R., Mikkonen T. (1992). Effects of density of young Scots pine (Pinus sylvestris) stand on moose (Alces alces) browsing. Acta Forestalia Fennica 231 article 7677. https://doi.org/10.14214/aff.7677.
Heiskanen J., Viiri H. (2005). Effects of mounding on damage by the European pine weevil in planted Norway spruce seedlings. Northern Journal of Applied Forestry 22: 154–161.
Heiskanen J., Saksa T., Luoranen J. (2013). Soil preparation method affects outplanting success of Norway spruce container seedlings on till soils susceptible to frost heave. Silva Fennica 47(1) article 893. https://doi.org/10.14214/sf.893.
Helle T., Pajuoja H., Nygrén K. (1987). Forest damage caused by moose and their economic value in Finland. Scandinavian Forest Economics 29: 7–26.
Henttonen H., Lilja A., Niemimaa J. (1994). Myyrien ja hyönteisten aiheuttamat sieni-infektiot koivun taimien uhkana. [Fungal infections caused by voles and insects as a threat to birch seedlings]. Metsäntutkimuslaitoksen tiedonantoja 496: 125–130. [In Finnish].
Holmsgaard E., Holstener-Jørgensen H., Yde-Andersen A. (1961). Bodenbildung, Zuwachs und Gesundheitszustand von Fichtenbeständen erster und zweiter Generation I Nord-Seeland. Det Forstlige Forsøgsvæsen i Danmark 27: 167.
Huitu O., Kiljunen N., Korpimäki E., Koskela E., Mappes T., Pietiäinen H., Pöysä H., Henttonen H. (2009). Density-dependent vole damage in silviculture and associated economic losses at a nationwide scale. Forest Ecology and Management 258(7): 1219–1224. https://doi.org/10.1016/j.foreco.2009.06.013.
Inward D.J.G., Wainhouse D., Peace A. (2012). The effect of temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and the potential impacts of climate change. Agricultural & Forest Entomology 14(4): 348–357. https://doi.org/10.1111/j.1461-9563.2012.00575.x.
Isomäki A., Kallio T. (1974). Consequences of injury caused by timber harvesting machines on the growth and decay of spruce (Picea abies (L.) Karst.). Acta Forestalia Fennica 136 article 7570. https://doi.org/10.14214/aff.7570.
Jactel H., Brockerhoff E., Duelli P. (2005). A test of the Biodiversity–Stability theory: meta-analysis of tree species diversity effects on insect pest infestations, and re-examination of responsible factors. In: Scherer-Lorenzen M., Körner C., Schulze E.D. (eds.). Forest diversity and function temperate and boreal systems. Springer. p. 235–262. https://doi.org/10.1007/3-540-26599-6_12.
Jactel H., Nicoll B.C., Branco M., Gonzalez-Olabarria J., Grodzki W., Långström B., Moreira F., Netherer S., Orazio C., Piou D., Santos H., Schelhaas M.J., Tojic K.l., Vodde F. (2009). The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science 66(7): 701. https://doi.org/10.1051/forest/2009054.
Jalkanen R. (1985). The occurrence and importance of Lophodermella sulcigena and Hendersonia acicola on Scots pine in Finland. Karstenia 25: 53–61.
Jalkanen R. (1986). Lophodermella sulcigena on Scots pine in Finland. Communicationes Instituti Forestalis Fenniae 136: 41.
Jalkanen R. (1990). Nitrogen fertilization as a cause of dieback of Scots pine at Paltamo, northern Finland. Aquilo Seria Botanica 29: 25–31.
Jalkanen R., Kurkela T. (1984). Männynversoruosteen aiheuttamat vauriot ja varhaiset pituuskasvutappiot. Summary: damage and early height growth losses caused by Melampsora pinitorqua. Folia Forestalia 587: 1–15.
Jalkanen R., Aalto T., Hallikainen V., Hyppönen M., Mäkitalo K. (2005). Viljelytaimikoiden hirvituhot Lapissa ja Kuusamossa. [Moose damage in seedling stands in Lapland and in Kuusamo]. Metsätieteen aikakauskirja 4/2005: 399–411. [In Finnish]. https://doi.org/10.14214/ma.6137.
Johansson K., Petterson N. (1996). Effect of initial spacing on biomass production, butt rot frequency and graded yield of Picea abies (L.) Karst. Forest & Landscape Research 1: 381–397.
Jokinen K. (1988). Metsänlannoituksen vaikutus puiden tuhonkestävyyteen: kirjallisuuskatsaus. Metsäntutkimuslaitoksen tiedonantoja 287. 57 p. ISBN 951-40-0823-5. [In Finnish].
Jokinen K., Tamminen P. (1979). Tyvilahoisten kuusikoiden jälkeen istutetuissa männyn taimistoissa esiintyvät sienituhot Keski-Satakunnassa. Fungal damage in young Scots pine stands replacing butt rot-infected Norway spruce stands in SW Finland. Folia Forestalia 399. 17 p.
Jonsson B.G., Brūmelis G.,Kuuluvainen T. (2011). Early classical studies of forest ecology in Northern Europe. Scandinavian Journal of Forest Research 26(S10): 1–2. https://doi.org/10.1080/02827581.2011.517946 .
Kaitaniemi P., Riihimäki J., Koricheva J., Vehviläinen H. (2007). Experimental evidence for associational resistance against the European pine sawfly in mixed tree stands. Silva Fennica 41(2): 259–268. https://doi.org/10.14214/sf.295.
Kallio T. (1965). Tutkimuksia maannousemasienen leviämisbiologiasta ja torjuntamahdollisuuksista Suomessa. Summary: studies on the biology of distribution and possibilities to control Fomes annosus in southern Finland. Acta Forestalia Fennica 78(3) article 7156. https://doi.org/10.14214/aff.7156.
Kallio T., Laine L. (1982). Der Einfluss forstlicher Intensivierungsmassnahmen auf die Pilzkrankheiten in finnischen Wäldern. Allgemeine Forstzeitschrift 8: 228–229.
Kallio T., Häkkinen R., Heinonen J. (1985). An outbreak of Gremmeniella abietina in central Finland. European Journal of Forest Pathology 15(4): 216–223. https://doi.org/10.1111/j.1439-0329.1985.tb00888.x.
Kärvemo S., Rogell B., Schroeder M. (2014). Dynamics of spruce bark beetle infestation spots: importance of local population size and landscape characteristics after a storm disturbance. Forest Ecology & Management 334: 232–240. https://doi.org/10.1016/j.foreco.2014.09.011.
Keča N., Solheim H. (2011). Ecology and distribution of Armillaria species in Norway. Forest Pathology 41(2): 120–132. https://doi.org/10.1111/j.1439-0329.2010.00644.x.
Klapwijk M.J., Bylund H., Schroeder M., Björkman C. (2016). Forest management and natural biocontrol of insect pests. Forestry 89(3): 253–262. https://doi.org/10.1093/forestry/cpw019.
Kolari K. (1983) (ed.). Growth disturbances of forest trees. Proceedings of international workshop and excursion held in Jyväskylä and Kivisuo, Finland, 10–13 October, 1982. Communicationes Instituti Forestalis Fenniae 116. 208 p.
Kollberg I., Bylund H., Schmidt A., Gershenzon J., Björkman C. (2013). Multiple effects of temperature, photoperiod and food quality on the performance of a pine sawfly. Ecological Entomology 38(2): 201–208. https://doi.org/10.1111/een.12005.
Korhonen K. (1978a). Intersterility groups of Heterobasidion annosum. Communicationes Instituti Forestalis Fenniae 94.6. 25 p.
Korhonen K. (1978b). Interfertility and clonal size in the Armillariella mellea complex. Karstenia 18: 31–42.
Korhonen K., Stenlid J. (1998). Biology of Heterobasidion annosum. In: Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (eds.). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. ISBN 0 85199 2757. p. 43–71.
Koricheva J., Vehviläinen H., Riihimäki J., Ruohomäki K., Kaitaniemi P., Ranta H. (2006). Diversification of tree stands as a means to manage pests and diseases in boreal forests: myth or reality? Canadian Journal of Forest Research 36(2): 324–336. https://doi.org/10.1139/x05-172.
Kuuluvainen T., Grenfell R. (2012). Natural disturbance emulation in boreal forest ecosystem management theories, strategies, and a comparison with conventional even-aged management. Canadian Journal of Forest Research 42(7): 1185–1203. https://doi.org/10.1139/x2012-064.
La Porta N., Capretti P., Thomsen I.M., Kasanen R., Hietala A.M., Weissenberg K. (2008). Forest pathogens with higher damage potential due to climate change in Europe. Canadian Journal of Plant Pathology 30(2): 177–195. https://doi.org/10.1080/07060661.2008.10540534.
Långström B., Day K.R. (2004). Damage, control and management of weevil pests, especially Hylobius abietis. In: Lieutier F., Day K.R., Battisti A., Grégoire J., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht. p. 415–444. https://doi.org/10.1007/978-1-4020-2241-8_19.
Larsson S., Tenow O. (1984). Areal distribution of a Neodiprion sertifer (Hym. Diprionidae) outbreak on Scots pine as elated to stand condition. Ecography 7(2): 81–90. https://doi.org/10.1111/j.1600-0587.1984.tb01108.x.
Lavsund S. (1987). Moose relationships to forestry in Finland, Norway and Sweden. Swedish Wildlife Research 1/1987(Suppl): 229–244.
Lavsund S., Nygrén T., Solberg E.J. (2003). Status of moose populations and challenges to moose management in Fennoscandia. Alces 39:109–130.
Leinonen R., Pöyry J., Söderman G., Tuominen-Roto L. (2016). Suomen yöperhosseuranta (Nocturna) 1993–2012. Suomen ympäristökeskuksen raportteja 15/2016. [In Finnish, abstract in English). https://helda.helsinki.fi/bitstream/handle/10138/161221/SYKEra_15_2016.pdf?sequence=1.
Lindberg M., Johansson M. (1992). Resistance of Picea abies seedlings to infection by Heterobasidion annosum in relation to drought stress. European Journal of Forest Pathology 22(2): 115–124. https://doi.org/10.1111/j.1439-0329.1992.tb01438.x.
Lindelöw A., Schröder M. (2008). The storm “Gudrun” and the spruce bark beetle in Sweden. Forstschutz Aktuell 44: 5–7.
Lindén M., Vollbrecht G. (2002). Sensitivity of Picea abies to butt rot in pure stands and in mixed stands with Pinus sylvestris in southern Sweden. Silva Fennica 36(4): 767–778. https://doi.org/10.14214/sf.519.
Lindner M., Maroschek M., Netherer S., Kremer A., Barbati A., Garcia-Gonzalo J., Seidl R., Delzon S., Corona P., Kolström M., Lexer M.J., Marchetti M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management 259(4): 698–709. https://doi.org/10.1016/j.foreco.2009.09.023.
Lumme I., Arkhipov V., Fedorets N., Mälkönen E. (1997). Männiköiden kunto Karjalan kannaksen-Kaakkois-Suomen ja Kostamuksen-Kainuun alueilla. Suomalais-venäläisen tutkimushankkeen loppuraportti. [The condition of pine stands in the Carelian Isthmus - Souht-eastern Finland and Kostamus-Kainuu regions. Final report of a Finnish-Russian co-operative project]. Metsäntutkimuslaitoksen tiedonantoja 665. 75 p. ISBN 951-40-1595-9. [In Finnish and Russian].
Luoranen J., Viiri H. (2012). Soil preparation reduces pine weevil (Hylobius abietis (L.)) damage on both peatland and mineral soil sites one year after planting. Silva Fennica 46(1): 151–161. https://doi.org/10.14214/sf.71.
Lyly O., Saksa T. (1992). The effect of stand density on moose damage in young Pinus sylvestris stands. Scandinavian Journal of Forest Research 7(1–4): 393–403. https://doi.org/10.1080/02827589209382732.
Lyytikäinen-Saarenmaa P., Tomppo E. (2002). Impact of sawfly defoliation on growth of Scots pine Pinus sylvestris (Pinaceae) and associated economic losses. Bulletin of Entomological Research 92: 137–140. https://doi.org/10.1079/ber2002154.
Marini L., Lindelöw Å., Jönsson A.M., Wulff S., Schroeder L.M. (2013). Population dynamics of the spruce bark beetle: a long-term study. Oikos 122(12): 1768–1776. https://doi.org/10.1111/j.1600-0706.2013.00431.x.
Markgren G. (1974). The moose in Fennoscandia. Le Naturaliste Canadien 101: 185–194.
Mattsson A. (2016). Reforestation challenges in Scandinavia. Reforesta 1: 67–85. https://doi.org/10.21750/refor.1.05.5.
Modig E., Magnusson B., Valinger E., Cedergren J., Lundqvist L. (2012). Damage to residual stand caused by mechanized selection harvest in uneven-aged Picea abies dominated stands. Silva Fennica 46(2): 267–274. https://doi.org/10.14214/sf.442.
Morrison D.J., Cruickshank M.G., Lalumière A. (2014). Control of laminated and Armillaria root diseases by stump removal and tree species mixtures: amount and cause of mortality and impact on yield after 40 years. Forest Ecology and Management 319: 75–98. https://doi.org/10.1016/j.foreco.2014.02.007.
Möykkynen T., Miina J. (2002). Optimizing the management of a butt-rotted Picea abies stand infected by Heterobasidion annosum from the previous rotation. Scandinavian Journal of Forest Research 17(1): 47–52. https://doi.org/10.1080/028275802317221073.
Müller M.M., Piri T., Hantula J. (2012). Ilmaston lämpeneminen haastaa nykyistä tehokkaampaan juurikäävän torjuntaan. [Climate warming demands more efficient control of root rot]. [In Finnish). Metsätieteen aikakauskirja 4/2012: 312–315. https://doi.org/10.14214/ma.6490.
Nevalainen S. (1999). Gremmeniella abietina in Finnish Pinus sylvestris stands in 1986–1992: a study based on the National Forest Inventory. Scandinavian Journal of Forest Research 14(2): 111–120. https://doi.org/10.1080/02827589950152836.
Nevalainen S., Matala J., Korhonen K., Ihalainen A., Nikula A. (2016). Moose damage in National Forest Inventories (1986–2008) in Finland. Silva Fennica 50(2) article 1410. https://doi.org/10.14214/sf.1410.
Niemelä P., Lindgren M., Uotila A. (1992). The effect of stand density on the susceptibility of Pinus sylvestris to Gremmeniella abietina. Scandinavian Journal of Forest Research 7(1–4): 129–133. https://doi.org/10.1080/02827589209382705.
Nikula A., Hallikainen V., Jalkanen R., Hyppönen M., Mäkitalo K. (2008). Modelling the factors predisposing Scots pine to moose damage in artificially regenerated sapling stands in Finnish Lapland. Silva Fennica 42(4): 587–603. https://doi.org/10.14214/sf.235.
Nilson K., Lundqvist L. (2001). Effect of stand structure and density on development of natural regeneration in two Picea abies stands in Sweden. Scandinavian Journal of Forest Research 16(3): 253–259. https://doi.org/10.1080/713785124.
Nilsson U., Luoranen J., Kolström T., Örlander G., Puttonen P. (2010). Reforestation with planting in northern Europe. Scandinavian Journal of Forest Research 25(4): 283–294. https://doi.org/10.1080/02827581.2010.498384.
Nordlander G., Hellqvist C., Johansson K., Nordenhem H. (2011). Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. Forest Ecology and Management 262(12): 2354–2363. https://doi.org/10.1016/j.foreco.2011.08.033.
Oliva J., Bendz-Hellgren M., Stenlid J. (2011). Spread of Heterobasidion annosum s.s. and Heterobasidion parviporum in Picea abies 15 years after stump inoculation. FEMS microbiology ecology 75(3): 414–429. https://doi.org/10.1111/j.1574-6941.2010.01020.x.
Parviainen J., Västilä S. (2011). State of Finland’s forests 2011 – based on the criteria and indicators of sustainable forest management. Publications of Ministry of Agriculture and Forestry 5a. 95 p.
Pautasso M., Holdenrieder O., Stenlid J. (2005). Susceptibility to fungal pathogens of forests differing in tree diversity. In: Scherer-Lorenzen M., Körner C., Schulze E.D. (eds.). Forest diversity and function temperate and boreal systems. Ecological Studies 176. Springer. p. 263–289. https://doi.org/10.1007/3-540-26599-6_13.
Peltola H., Kellomäki S., Väisänen H., Ikonen V.-P. (1999). A mechanistic model for assessing the risk of wind and snow damage to single trees and stands of Scots pine, Norway spruce, and birch. Canadian Journal of Forest Research 29(6): 647–661. https://doi.org/10.1139/cjfr-29-6-647.
Peltola H., Gardiner B., Nicoll B. (2013). Mechanics of wind damage. In: Gardiner B., Schuck A., Schelhaas M., Orazio C., Blennow K., Nicoll B., (eds.). What science can tell us? “Living with storms damage to forests”. European Forest Institute. p. 31–38.
Pettersson M., Rönnberg J., Vollbrecht G., Gemmel P. (2003). Effect of thinning and Phlebiopsis gigantea stump treatment on the growth of Heterobasidion parviporum inoculated in Picea abies. Scandinavian Journa of Forest Research 18(4): 362–367. https://doi.org/10.1080/02827580310007845.
Petersson M., Örlander G., Nordlander G. (2005). Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry 78(1): 83–92. https://doi.org/10.1093/forestry/cpi008.
Piri T. (2003). Early development of root rot in young Norway spruce planted on sites infected by Heterobasidion in southern Finland. Forest Pathology 33(4): 604. https://doi.org/10.1139/x02-200.
Piri T., Korhonen K. (2001). Infection of advance regeneration of Norway spruce by Heterobasidion parviporum. Canadian Journal of Forest Research 31(6): 937–942. https://doi.org/10.1139/x01-021.
Piri T., Korhonen K. (2007). Spatial distribution and persistence of Heterobasidion parviporum genets on a Norway spruce site. Forest Pathology 37(1): 1–8. https://doi.org/10.1111/j.1439-0329.2007.00482.x.
Piri T., Korhonen K. (2008). The effect of winter thinning on the spread of Heterobasidion parviporum in Norway spruce stands. Canadian Journal of Forest Research 38(10): 2589–2595. https://doi.org/10.1139/x08-103.
Piri T., Korhonen K., Sairanen A. (1990). Occurrence of Heterobasidion annosum in pure and mixed spruce stands in Southern Finland. Scandinavian Journal of Forest Research 5(1–4): 113–125. https://doi.org/10.1080/02827589009382598.
Piri T., Valkonen S. (2013). Incidence and spread of Heterobasidion root rot in uneven-aged Norway spruce stands. Canadian Journal of Forest Research 43(9): 872–877. https://doi.org/10.1139/cjfr-2013-0052.
Pouttu A., Annila E. (2010). Kirjanpainajalla kaksi sukupolvea kesällä 2010. [Ips typoographus had two generations in 2010]. Metsätieteen aikakauskirja 4/2010: 521–523. [In Finnish]. https://doi.org/10.14214/ma.6951.
Pukkala T., Laiho O., Lähde E. (2016). Continuous cover management reduces wind damage. Forest Ecology & Management 372: 120–127. https://doi.org/10.1016/j.foreco.2016.04.014.
R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
Read D.J. (1968). Some aspects of the relationship between shade and fungal pathogenity in an epidemic disease of pines. New Phytologist 67(1): 39–48. https://doi.org/10.1111/j.1469-8137.1968.tb05452.x.
Rennerfelt E. (1946). Om rotrötan (Polyporus annosus) i Sverige. Dess utbredning och sätt att uppträda. Referat: Über die Wurzelfäule in Sweden. Ihre Verbreitung und ihr Vorkommen unter verschiedenen Verhältnissen. Meddelanden från Statens Skogsforskningsinstitut 35: 1–88.
Rikala R. (2004). Summary. In: Rikala R. (ed.). Growth disturbances in trees on fertile mineral soils. Final report of the project “Growth disturbances in burnt-over Norway spruce stands”. Finnish Forest Research Institute Research Papers 934: 5–19. http://www.metla.fi/julkaisut/mt/2004/934.htm.
Rishbeth J. (1978). Infection foci of Armillaria mellea in first-rotation hardwoods. Annals of Botany 42: 1131–1139.
Rönnberg J., Petrylaite E., Nilsson G., Pratt J. (2006). Two studies to assess the risk to Pinus sylvestris from Heterobasidion spp. in southern Sweden. Scandinavian Journal of Forest Research 21(5): 405–413. https://doi.org/10.1080/02827580600917379.
Rönnberg J., Berglund M., Johansson U. (2007). Incidence of butt rot at final felling and at first thinning of the subsequent rotation of Norway spruce stands in south-western Sweden. Silva Fennica 41(4): 639–647. https://doi.org/10.14214/sf.272.
Schelhaas M., Nabuurs G., Schuck A. (2003). Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biology 9(11): 1620–1633. https://doi.org/10.1046/j.1365-2486.2003.00684.x.
Schmidt R.A. (1978). Diseases in forest ecosystems. The importance of functional diversity. In: Horsfall J.G., Cowling E.B. (eds.). Plant disease. An advanced treatise, vol II. How disease develops in populations. Academic Press, New York. p. 287–315. https://doi.org/10.1016/b978-0-12-356402-3.50022-0.
Shorohova E., Fedorchuk V., Kuznetsova M., Shvedova O. (2008). Wind-induced successional changes in pristine boreal Picea abies forest stands: evidence from long-term permanent plot records. Forestry 81(3): 335–359. https://doi.org/10.1093/forestry/cpn030.
Siren M., Hyvonen J., Surakka H. (2015). Tree damage in mechanized uneven-aged selection cuttings. Croatian Journal of Forest Engineering 36: 33–42.
Stenlid J. (1987). Controlling and predicting the spread of Heterobasidion annosum from infected stumps and trees of Picea abies. Scandinavian Journal of Forest Research 2(1–4): 187–198. https://doi.org/10.1080/02827588709382457.
Subramanian N., Bergh J., Johansson U., Nilsson U., Sallnäs O. (2016). Adaptation of forest management regimes in southern Sweden to increased risks associated with climate change. Forests 7(1), 8. https://doi.org/10.3390/f7010008.
Surakka H., Sirén M., Heikkinen J., Valkonen S. (2011). Damage to saplings in mechanized selection cutting in uneven-aged Norway spruce stands. Scandinavian Journal of Forest Research 26(3): 232. https://doi.org/10.1080/02827581.2011.552518.
Therneau T., Atkinson B., Ripley B. (2015). rpart: Recursive Partitioning and Regression Trees. R package version 4.1-10. https://CRAN.R-project.org/package=rpart.
Uotila A. (1985). Siemenen siirron vaikutuksesta männyn versosyöpäalttiuteen Etelä- ja Keski-Suomessa. Summary: on the effect of seed transfer on the susceptibility of Scots pine to Ascocalyx abietina in southern and central Finland. Folia Forestalia 639. 12 p.
Valinger E., Pettersson N. (1996). Wind and snow damage in a thinning and fertilization experiment in Picea abies in southern Sweden. Forestry 69(1): 25–34. https://doi.org/10.1093/forestry/69.1.25.
Vanhanen H., Veteli T.O., Päivinen S., Kellomäki S., Niemelä P. (2007). Climate change and range shifts in two insect defoliators: gypsy moth and nun moth – a model study. Silva Fennica 41(4): 621–638. https://doi.org/10.14214/sf.469.
von Sydow F. (1997). Abundance of pine weevils (Hylobius abietis) and damage to conifer seedlings in relation to silvicultural practices. Scandinavian Journal of Forest Research 12(2): 157–167. https://doi.org/10.1080/02827589709355397.
von Weissenberg K. (1990). Värd-parasitförhållanden i skogliga ekosystem. En litteraturstudie. Silva Fennica 24(1): 129–139. https://doi.org/10.14214/sf.a15567.
Wermelinger B. (2004). Ecology and management of the spruce bark beetle Ips typographus – a review of recent research. Forest Ecology and Management 202(1–3): 67–82. https://doi.org/10.1016/j.foreco.2004.07.018.
Virjamo V., Julkunen-Tiitto R., Henttonen H., Hiltunen E., Karjalainen R., Korhonen J., Huitu O. (2013). Differences in vole preference, secondary chemistry and nutrient levels between naturally regenerated and planted Norway spruce seedlings. Journal of chemical ecology 39(10): 1322–1334. https://doi.org/10.1007%2Fs10886-013-0352-6.
Virtanen T., Neuvonen S., Nikula A., Varama M., Niemelä P. (1996). Climate change and the risks of Neodiprion sertifer outbreaks on Scots pine. Silva Fennica 30(2–3): 169–177. https://doi.org/10.14214/sf.a9229.
Vollbrecht G., Agestam E. (1995). Modelling incidence of root rot in Picea abies plantations in Southern Sweden. Scandinavian Journal of Forest Research 10(1–4): 74–81. https://doi.org/10.1080/02827589509382870.
Zeng H., Peltola H., Kellomäki S. (2007). Interactive clear-cut regime selection for risk management of wind damage using reference point approach. Ecological Informatics 2(2): 150–158. https://doi.org/10.1016/j.ecoinf.2007.04.002.
Total of 137 references.


