Differences in root growth of Quercus ilex and Quercus suber seedlings infected with Phytophthora cinnamomi
León I., García J. J., Fernández M., Vázquez-Piqué J., Tapias R. (2017). Differences in root growth of Quercus ilex and Quercus suber seedlings infected with Phytophthora cinnamomi. Silva Fennica vol. 51 no. 4 article id 6991. https://doi.org/10.14214/sf.6991
Highlights
- Root growth of two Quercus sp. differs significantly after infection with Phytophthora cinnamomi
- We observed a marked decrease in the growth of new roots in Quercus ilex with increasing inoculum level
- Roots were longer but thinner with a moderate inoculum level in Quercus suber.
Abstract
In the southwest of the Iberian Peninsula, Phytophthora cinnamomi Rands is causing irreversible damage to populations of the two most common species of Quercus, the holm oak (Quercus ilex L.) and the cork oak (Quercus suber L.). Although the symptoms are similar in the two species, the mortality rates are different. We found significant differences in the post-infection growth of the root system as a function of tree species, as well as initial plant size, and inoculum level. We observed a marked decrease in the growth of new roots in Q. ilex with increasing inoculum level, while in Q. suber, we found longer but thinner roots with a moderate inoculum level. In both species, we observed a worsening in the water status of the plants from the lowest inoculum level.
Keywords
root rot;
holm oak;
cork oak;
infection;
inoculum level;
oaks decline
- León, University of Huelva, Agroforestry department, Calle Dr. Cantero Cuadrado, 6, 21004 Huelva, Spain E-mail isabel.leon@dcaf.uhu.es
- García, University of Huelva, Agroforestry department, Calle Dr. Cantero Cuadrado, 6, 21004 Huelva, Spain E-mail juanjose.garcia@dcaf.uhu.es
- Fernández, University of Huelva, Agroforestry department, Calle Dr. Cantero Cuadrado, 6, 21004 Huelva, Spain E-mail nonoe@uhu.es
- Vázquez-Piqué, University of Huelva, Agroforestry department, Calle Dr. Cantero Cuadrado, 6, 21004 Huelva, Spain E-mail jpique@dcaf.uhu.es
-
Tapias,
University of Huelva, Agroforestry department, Calle Dr. Cantero Cuadrado, 6, 21004 Huelva, Spain
E-mail
rtapias@uhu.es

Received 6 March 2017 Accepted 6 September 2017 Published 15 September 2017
Views 94841
Available at https://doi.org/10.14214/sf.6991 | Download PDF

1 Introduction
Since the beginning of the 20th century, there has been a decrease in forest cover in Mediterranean ecosystems (Brasier 1992). This decline reflects the great susceptibility to infection of Iberian trees of Quercus forests (Moralejo et al. 2009). Since the beginning of the nineteen-eighties, oak decline has been observed in the Iberian Peninsula as well as other parts of the world, and this decline is considered a complex syndrome involving many factors (Anselmi et al. 2005). This phenomenon is regarded as serious in the southwest of the Iberian Peninsula, it being reported that the trees are affected by a root disease caused by the plant-pathogenic oomycete species Phytophthora cinnamomi Rands (Gallego et al.1999; Sánchez et al. 2002).
Worldwide, P. cinnamomi is having a major impact on a wide range of forests and tree crops of several species: Pinus spp., Eucalyptus spp. and Quercus spp. (Podger 1972; Batini et al. 1974; Malajczuk et al. 1977; Cahill et al. 1993; Serrano et al. 2012b; Eggers et al. 2012). Infecting a wide variety of host species, this pathogen has been confirmed to be the main agent involved in the impoverishment of Quercus forests in the southeast of the Iberian Peninsula (Brasier et al. 1993; Gallego et al. 1999; Sánchez et al. 2002; Camilo-Alves et al. 2013). Specifically, various studies have shown that the presence of P. cinnamomi in Quercus ilex L. and Quercus suber L. weakens their root systems (Moreira and Martins 2005). One of the first effects of this weakening is dieback of fine absorbing roots, impairing their function and contributing to a progressive deterioration of the tree health, leading to plant death in most cases (Brasier et al. 1993; Horta et al. 2010).
When P. cinnamomi enters the host, it multiplies rapidly, producing new infectious zoospores that reach neighbouring roots when the soil is water saturated (Horta et al. 2010). Young actively growing plants with a high proportion of absorbing roots are particularly sensitive, as this type of root is the most affected (Marçais et al. 1996). Hence, measurements related to secondary roots are considered to be effective for detecting infection by this pathogen (Robin et al. 2001) and exploring differences in its effect between species, populations or genotypes (Cahill et al. 1993; Belisario et al. 2009). At the time of infection, a high density of inoculum is a necessary condition; in addition, infection depends on various environmental factors (Moralejo et al. 2009).
Further, the development of P. cinnamomi infection is influenced by the susceptibility of the host species (Serrano et al. 2012a). Several studies have shown that this pathogen aggressively attacks Q. ilex and Q. suber (Rodriguez Molina et al. 2002; Moralejo et al. 2009; Serrano et al. 2012b; Sánchez et al. 2005), while it does not seem to cause severe lesions in other Quercus species (Marçais et al. 1996). In general, inoculating Quercus species with this pathogen induces a reduction in leaf water potential and losses in active secondary roots (Robin et al. 2001; Luque et al. 1999), but the susceptibility of the root system to the disease varies by host species (Moralejo et al. 2009).
In this context, the aim of our study was to investigate differences in the symptoms of disease due to infection by P. cinnamomi between Q. ilex and Q. suber. To achieve this, we inoculated seedlings of these two species with a mycelial suspension of the pathogen, and assessed the root regeneration potential and plant water status under nursery conditions. The primary objective of this study was to explore the potential usefulness of a set of quantitative variables in improving our ability to detect differences in susceptibility between the species. Secondary objectives were to assess differences in responses in terms of rate of survival and new root production between tree species.
2 Materials and methods
2.1 Plant material
The vegetal material studied was grown from acorns collected in Huelva province from October to December 2008 from 10 trees of each species, Q. ilex and Q. suber, in an area affected by oak decline in the southwest of the Iberian Peninsula. To collect the acorns, we set a minimum distance of 100 m between trees to reduce the chance of using individuals that were closely genetically related (Tapias et al. 2004). At least 300 acorns from each tree were collected, washed and disinfected with bleach (NaClO) (0.1% v/v). These were stored in the dark at 4 °C until use. At the beginning of February 2009, the acorns were laid on trays of damp perlite for pre-germination (Andivia et al. 2013), the aim being to shorten the germination period and achieve greater uniformity in germination across the sample. Fifteen days later, almost all the acorns had germinated. We randomly selected 200 acorn sprouts per tree transplanted them into 330 cm3 Superleach® Bardi containers containing a peat-vermiculite mixture (3/1 v/v) (Tapias et al. 2004). Seedlings from the two species were randomly distributed into different trays, which were placed in semi-shade (under 50% shade cloth). Trays were watered continuously as required and regularly moved during the growth cycle to minimise positioning effects (Tapias et al. 2004).
2.2 Pathogen material
After a year of growth, in March 2010, seedlings were inoculated by application of a water suspension of chlamydospores and mycelia (Sánchez et al. 2001) to the root. To achieve effective infection (Eggers et al. 2012), the inoculum contained three isolates of P. cinnamomi (P37, P45, and P203). These were previously held in the Department of Agroforestry Sciences at the University of Huelva and characterised by the Unit of Agricultural Sciences and Technologies at the University of Algarve (Faro) and Aeeiro Plant Pathology Station (Pontevedra). Mycelia of each of the three strains were first grown in Petri dishes in V8 juice agar medium (Miller 1955) in the dark at 20 °C for 1 month. Then, once separated from the culture medium, they were blended with distilled water. To determine the appropriate inoculum level to produce symptoms in the seedlings, we produced sets of solutions containing different concentrations of active P. cinnamomi mycelia as well as the control. Inoculum level 1 (dose 1, D1), inoculum level 2 (dose 2, D2) and inoculum level 3 (dose 3, D3) were solutions containing, respectively, the mycelia from 1.5 Petri dishes (half the mycelia grown in a Petri dish per strain for each of the aforementioned strains of the pathogen), 6 Petri dishes (2 dishes per strain), and 24 Petri dishes (8 dishes per strain); in each case, the mycelia were mixed in 300 ml of distilled water. Before use, the mixture was stirred and, for each inoculum level, 10 ml of solution of the infectious inoculum was injected into the root ball of each seedling, in three places to ensure a broad distribution of the mixture (Horta et al. 2010).
2.3 Experimental design
With the goal of quantifying the development of the root system, after inoculation, for each species, batches of 20 randomly-selected seedlings per inoculum level were transplanted into 45-l expanded polystyrene boxes (40 × 49 cm) containing wet perlite. This experiment was performed in duplicate. Humidity and temperature were controlled (relative humidity >70%; air temperature of 30–35 °C) throughout the period of the trial. To favour infection and the production of zoospores, seedlings were exposed to flood-drought cycles (water saturation up to 5 cm from the top of the box for 2 days, followed by the drainage of excess water and a gap of 5 days, without causing severe stress) (Sánchez et al. 2001). As the boxes were made of an opaque impermeable material, the water level inside was estimated using the principle of communicating vessels, with an external system of transparent tubes, connected to each box.
2.4 Symptom assessment
After 45 days of exposure to flood-drought cycles, we selected 15 seedlings per species and treatment to assess root regeneration potential, using quantitative and qualitative parameters to describe: the size of the new roots developed before the infection; the production of roots since the infection, the pathogen tending to colonize roots around the periphery of the root ball; and the health status of the root system.
Initially, we cut free and washed the new secondary roots produced around the outside of the root ball in the perlite bed, and determined the new root dry weight (NRW) and length (NRL). We then removed the root ball, and washed the roots carefully with running water. Once the root system was free of soil, we separated, dried and weighed the secondary roots (SRW) and the primary root (PRW), contained in the root ball and produced before the infection, and also measured the length of the primary root (PRL). To determine the dry weights, the roots and above-ground biomass were oven dried at 80 °C to constant weight, this taking 2 days. We calculated the weight-to-length (W/L) ratio for new roots to assess their thickness. Regarding the above-ground parts of the seedlings, we weighed the dried main stem (including leaves) (MSW) and measured its length (MSL). Then, we counted the number of leaves (NL) on each seedling. We calculated the total weight of the seedling (TW) as the sum of MSW + PRW + SRW, reflecting the initial seedling size. All weights were measured in grams with a precision balance and all lengths in centimetres with a meter stick.
In addition, we made a visual assessment of leaf damage and defoliation on each seedling using the classification described in Sánchez et al. (2001). Seedlings with 0to 10% of leaf damage were categorised as class 0; 11 to 25% as class 1; 26 to 75% as class 2; 76 to 90% as class 3, and over 90%, including plants without foliage, as class 4.
On the other hand, we measured the pre-dawn xylem water potential (Ψ) for each seedling using a pressure chamber at the end of the experiment for Q. ilex and Q. suber seedlings (Model 1000, PMS Instruments, Corvallis, OR, USA). For this, we selected two leaves from each seedling (all from the middle of the plant) in the morning before they were exposed to direct sunlight. These were cut and kept in the dark, in plastic bags in a fridge, for delivery to the laboratory, and measurements were taken for all leaves within 30 minutes of when they were cut (Carevic et al. 2010).
2.5 Pathogen reisolation
To confirm that infection had indeed developed in the seedlings inoculated with P. cinnamomi, we obtained 2-cm long fragments of dead roots and washed them for 4 h under running water. The dead roots were cut into 3–4 mm segments, with sterile scalpels, and placed in Petri dishes containing 20 cm3 of NARPH (Nistatine-Ampiciline, Rifampicin, Penta cloro nitro benzene, Himexazol) selective medium (Horta et al. 2010). For each of the treatment groups and for the control, 60 fragments (in 10 Petri dishes each containing 6 fragments) were cultured in the dark at 25 °C for 4 days.
2.6 Data analysis
After assessing the correlations between pairs of study variables, we carried out a two-factor analysis of variance (ANOVA) with NRW, NRL, W/L ratio and Ψ as dependent variables and species and inoculum level as independent variables. We considered TW as a covariate, to take into account the initial size of the seedling, and species (S) and inoculum level (D) as fixed factors. When the ANOVA results were significant, we performed Tukey’s test for multiple comparisons, with a significance level of 5% (p < 0.05).
For each species, we assessed the effect of each inoculum level on the root regeneration potential by comparing the slopes of the regressions between the dependent variables, NRW and NRL, and seedling size (TW).
Model: Y = Inoculum level × TW(covariable) + error
For both types of analysis, we checked the normality assumption (Kolmogorov-Smirnov test) and the homoscedasticity of the data (Levene´s test). Statistical analysis of the data was carried out with the linear regression procedure in IBM SPSS Statistics for Windows (Version 19.0. Armonk, NY).
3 Results
After one growing season (seedlings in their first season), there were differences in size between the two species (Table 1). In Q. suber, the stems and primary roots were twice as long and heavy as those of Q. ilex, and there were also 35% more secondary roots within the root ball. These parameters did not change significantly during the infection trial. All the variables related to the initial seedling size, both for the above- and below-ground parts, were positively correlated (p < 0.001), and hence analysis focused on the variables associated with root regeneration.
| Table 1. Comparison of the mean values (±SE) of secondary root, primary root and the main stem weights (SRW, PRW and MSW, respectively) and main stem length (MSL), visual above-ground assessment (VA); and water potential (Ψ) in Quercus ilex and Quercus suber control seedlings (n = 45) 45 days after inoculation. | ||||||
| SRW (g) | PRW (g) | MSW (g) | MSL (cm) | VA (class) | Ψ (-MPa) | |
| Quercus ilex | 0.319 ± 0.025 | 1.547 ± 0.176 | 0.081 ± 0.062 | 10.55 ± 0.75 | 1.60 ± 0.21 | 0.76 ± 0.19 |
| Quercus suber | 0.434 ± 0.047 | 3.984 ± 0.467 | 2.041 ± 0.197 | 23.06 ± 2.017 | 2.00 ± 0.17 | 0.22 ± 0.03 |
| p-value | 0.04 | 0.006 | <0.001 | <0.001 | 0.001 | 0.013 |
Forty-five days after infection, we observed significant differences between the two species in the rate of production of new roots (around the original root ball), both under control (non-inoculated) and infection (inoculated) conditions (Figs. 1–3). In control seedlings, we found differences in NRW, with weights of 0.02 ± 0.00 g in Q. ilex and 0.10 ± 0.03 g in Q. suber (p < 0.001), and also in NRL, new roots being significantly longer in Q. suber (29.76 ± 4.58 cm, n = 15) than Q. ilex (18.84 ± 3.72 cm, n = 15) (p < 0.001).
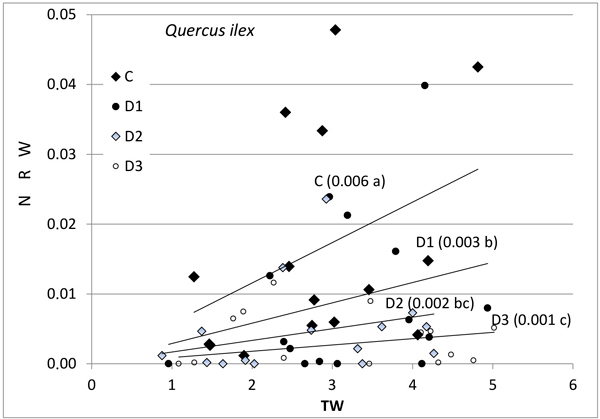
Fig. 1. Relationship between new root weight (g) (NRW) and total seedling weight (g) (TW) in Quercus ilex for the different Inoculum level used. Different letters by the regression lines indicate significantly different slopes. C (control): solid diamond; D1 (dose 1): solid circle; D2 (dose 2): hollow diamond; and D3 (dose 3): hollow circle.
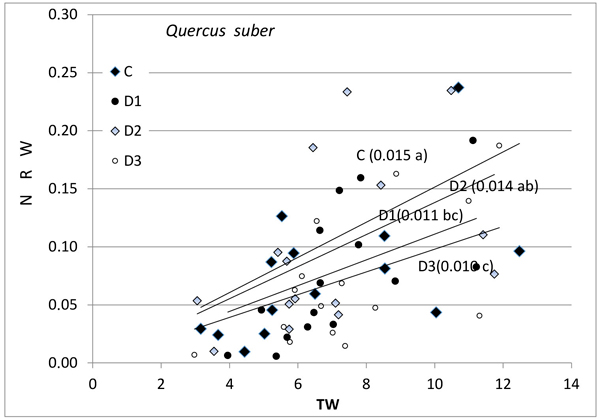
Fig. 2. Relationship between new root weight (g) (NRW) and total seedling weight (g) (TW) in Quercus suber for the different Inoculum level used. Different letters by the regression lines indicate significantly different slopes. C (Control): solid diamond; D1 (dose 1): solid circle; D2 (dose 2): hollow diamond; and D3 (dose 3): hollow circle.
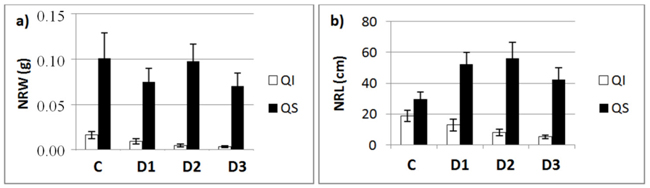
Fig. 3. a) New root weight (NRW; mean ± SD) and b) new root length (NRL; mean± SD) for each of study Inoculum level (C, control; D1, dose 1; D2, dose 2 and D3, dose 3) in seedlings of Quercus ilex (Qi) and Quercus suber (Qs) (n = 45).
Further, the production of new roots was associated with seedling size and with inoculum level (Figs. 1–3), but the relationship was different for weight and length and varied between the species. No differences in response between species were found for control seedlings. NRW was correlated with seedling size (TW) both for Q. ilex (Fig. 1, p = 0.006) and Q. suber (Fig. 2, p = 0.001). Considering all treatments, the significance decreases with increasing inoculum level (as the slope of the regression line decreases). In Q. suber, both NRW and NRL were associated with seedling size and inoculum level but in different ways. The slope of NRW vs TW decreases with inoculum level (Fig. 2); however, the slope of NRL vs TW increases with inoculum level (4.07 ± 0.94 b; 7.55 ± 0.94 a; 7.78 ± 0.94 a and 5.61 ± 0.89 b cm g–1 for C, D1, D2 and D3 respectively). On the other hand, in Q. ilex, the slopes of NRW (Fig. 1) and NRL (6.40 ± 0.93 a; 4.12 ± 0.83 b; 3.09 ± 0.96 bc and 1.55 ± 0.81 c cm g–1 for C, D1, D2 and D3 respectively) versus TW both decrease with inoculum level.
Fig. 3 shows the mean values of NRW and NRL for the two species as a function of inoculum level. These data confirm the results of the slope analysis, i.e., the two species behave differently in terms of length and weight. NRW decreases with increasing inoculum level in both species, while the length of new roots outside the root ball (NRL) in inoculated seedlings decreased in the case of Q. ilex, but increased in the case of Q. suber (Fig. 3b).
In Q. ilex, we did not find significant differences in W/L ratio between controls (8 10–4 ± 1 10–4 g cm–1) and inoculated seedlings (6 10–4 ± 1 10–4 g cm–1). In contrast, for Q. suber, differences were significant (p < 0.001), the ratio being higher for controls (3 10–4 ± 2 10–4 g cm–1) than inoculated seedlings (1 10–4 ± 2 10–4 g cm–1) (p < 0.001).
The inoculum level also affected the position of the new roots with respect to the root ball, the pattern varying between species. In controls, we found a proliferation of new roots all around the root ball in both species. In contrast, in inoculated seedlings, we found root proliferation in Q. suber all over the root ball, whereas Q. ilex only developed new roots around the upper half of the root ball, that is, the portion close to the surface during the flood-drought treatment.
No significant differences were detected in either of the species by visual above-ground assessment of the seedling (Fig. 4). On the other hand, root necrosis followed the expected pattern. That is, the root system was increasingly severely affected as the inoculum level increased in both species.
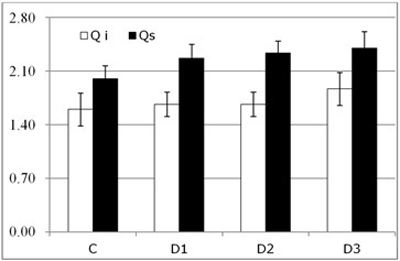
Fig. 4. Results of visual above-ground assessment (symptom score, mean ± SD) for each of the study Inoculum level (C, control; D1, dose 1; D2, dose 2 and D3, dose 3) in seedlings of Quercus ilex (Qi) and Quercus suber (Qs) (n = 120).
In terms of xylem water potential at the end of the trial, Ψ, we did not find any significant differences between the species (Fig. 5), both having a similar pattern of response. We did, however, find dramatic and significant differences in water potential between control and inoculated seedlings (p < 0.001).
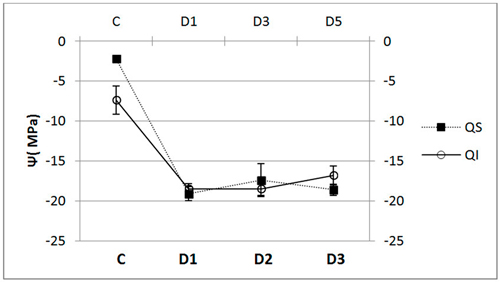
Fig. 5. Water potential (Ψ, mean ± SD) for each of the study Inoculum (C, control; D1, dose 1; D2, dose 2 and D3, dose 3) in seedlings of Quercus ilex (Qi) (n = 60) and Quercus suber (Qs) (n = 60).
P. cinnamomi was reisolated from samples of necrotic absorbing root tissue in 75% to 95% of cases in both species in inoculated plants. As would be expected, the pathogen was not observed in any cultures from control roots.
4 Discussion
Analysis of root regeneration potential is an effective methodology for assessing differences in susceptibility between species under controlled inoculation with P. cinnamomi. Differences in root regeneration potential have previously been found in research on cold tolerance (Gratani et al. 2003), drought resistance or the effect of different watering regimens (Ogaya and Peñuelas 2003; Andivia et al. 2013), and fertilization (Gimeno et al. 2009). In our study, we firstly explored a set of quantitative variables that might help to detect differences in susceptibility between species, populations, families or individuals, improving on analysis based on qualitative variables (survival or degree of damage) used in most studies (Sánchez et al. 2001; Tapias et al. 2004; Horta et al. 2010). We found that root regeneration potential, together with water potential, reveals significant differences in short periods of time, and hence could be used for early selection.
Secondly, our research suggests that the infection by P. cinnamomi has a different effect on the two Quercus species studied. In general, we confirm the symptoms of the disease as described in numerous previous studies (Brasier et al. 1993; Sánchez et al. 2001; Tapias et al. 2004; Sghaier-Hammami et al. 2013), namely, decreases in the production of new roots, in terms of weight and length, as well as in the development of functional roots during growth. Dieback of absorbing roots is also observed followed by root necrosis (Widmer et al. 2012), and as a consequence, there is a loss in plant vigour, leaf damage and defoliation (Horta et al. 2010). Notably, however, regarding the symptoms observed in the roots, we found differences between the species. In the Q. ilex seedlings inoculated with P. cinnamomi, there was a sharp reduction in the development of new roots, as shown in other studies (Robin et al. 2001; Moralejo et al. 2009). In contrast, after inoculation, Q. suber seedlings developed a larger number of secondary roots; these were longer, but thinner than those in control and had a lower overall weight. The symptoms of root necrosis and damage to the above-ground parts of the seedling 45 days after the inoculation are similar to those observed by Horta et al. (2010) over longer periods of time.
Overall, our data suggest that, unlike Q. ilex, Q. suber has mechanisms for responding to infection that allow it to develop even with high levels of infection. Evidence of differences in response supports the view that Q. suber is more tolerant to the disease than Q. ilex. This view is consistent with the first susceptibility studies on these species after root inoculation of P. cinnamomi (Brasier et al. 1993; Robin and Desprez-Loustau 1998; Sánchez et al. 2001).
We found significant differences in pre-dawn leaf water potential between control and inoculated seedlings. Leaf water potential in control seedlings of both species was similar to that reported in other studies on trees of similar age with good growth despite moderate levels of stress (Moralejo et al. 2009). Infection seems to trigger a decrease in leaf water potential, inoculated seedlings having lower values than controls. Hence, we confirm that infection by P. cinnamomi is associated with a reduction in water potential in both Q. ilex (Robin et al. 2001) and Q. suber (Luque et al. 1999). Other authors suggest that the stress suffered by seedlings due to flood-drought cycles also contributes to the reduction in water potential, although to a lesser extent than infection (Robin et al. 2001). On the basis of our results, however, we refute the idea that the decrease in water potential is exclusively due to water stress due to flood/drought cycles as indicated by other authors (Dawson and Weste 1984), and instead, attribute it to a marked deterioration of the root system.
Our results are similar to those of Marçais et al. (1996) who reported different responses to P. cinnamomi in Quercus and Castanea species. Specifically, in Quercus, the pathogen most severely affects the primary root, while secondary roots are more resistant.
Root necrosis caused by infection results in a decrease in water absorption, eventually affecting the above-ground parts causing leaf yellowing, damage and death (Brasier et al. 1993). In this study, we have observed the initial effects of infection, the lack of damage to woody roots or above-ground parts of the seedling being attributable to the short duration of the study.
To conclude, the response mechanisms to infection by P. cinnamomi seem to be different in the two species under study. Specifically, the greater susceptibility of Q. ilex compared to Q. suber is closely related to its root regeneration potential.
Acknowledgements
This research was funded by the Andalusian Regional Ministry of Science, Innovation and Business (project P07-RNM-03108), the European Social Fund and European Regional Development Fund. We also thank participants from CEI CamBio for their support.
References
Andivia E., Vázquez-Piqué J., Fernández M., Alejano R. (2013). Litter production in Holm oak trees subjected to different pruning intensities in Mediterranean dehesas. Agroforestry Systems 87(3): 657–666. https://doi.org/10.1007/s10457-012-9586-5.
Anselmi N., Mazzaglia A. (2005). Correlation between the incidence of endophytic pathogenic fungiand oak decline in Quercus ilex L. after fire damages. IOBC/WPRS Bulletin 28: 93–99.
Batini F.E. (1974). Susceptibility os Eucalyptus marginata and Eucaliptus calophylla seedlings to infection by Phytophthora cinnamomi in nutrients solution. Forests Department, Western Australia, Research Paper 14.
Belisario A., Galli M., Wajnberg E. (2009). Evaluation of Juglans species for resistance to Phytophthora cinnamomi: differences in isolate virulence and response to fosetyl-Al. Forest Pathology 39(3): 168–176. https://doi.org/10.1111/j.1439-0329.2008.00573.x.
Brasier C.M. (1992). Oak tree mortality in Iberia. Nature 360(6404): 539–539. https://doi.org/10.1038/360539a0.
Brasier C.M., Robredo F., Ferraz J.F. (1993). Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathology 42(1): 140–145. https://doi.org/10.1111/j.1365-3059.1993.tb01482.x.
Cahill D.M., Bennett I.J., McComb J.A. (1993). Mechanisms of resistance to Phytophthora cinnamomi in clonal, micropropagated Eucaliptus marginata. Plant Pathology 42(6): 865–872. https://doi.org/10.1111/j.1365-3059.1993.tb02672.x.
Camilo-Alves C.S.P., Esteves da Clara M.I., Cabral de Almeida Ribeiro N.M. (2013). Decline of Mediterranean oak trees and its association with Phytophthora cinnamomi: a review. European Journal of Forest Research 132(3): 411–432. https://doi.org/10.1007/s10342-013-0688-z.
Carevic F.S., Fernández M., Alejano R., Vázquez-Piqué J., Tapias R., Corral E., Domingo J. (2010). Plant water relations and edaphoclimatic conditions affecting acorn production in a holm oak (Quercus ilex L. ssp. ballota) open woodland. Agroforestry Systems 78(3): 299–308. https://doi.org/10.1007/s10457-009-9245-7.
Dawson P., Weste G. (1984). Impact of root infection by Phytophthora cinnamomi on the water relations of two Eucalyptus species that differ in susceptibility. Phytopathology 74(4): 486–490. https://doi.org/10.1094/Phyto-74-486.
Eggers J.E., Balci Y., MacDonald W.L. (2012). Variation among Phytophthora cinnamomi isolates from oak forest soils in the Eastern United States. Plant Disease 96(11): 1608–1614. https://doi.org/10.1094/PDIS-02-12-0140-RE.
Gallego F.J., de Algaba A.P., Fernández Escobar R. (1999). Etiology of oak decline in Spain. European Journal of Forest Pathology 29: 17–27. https://doi.org/10.1046/j.1439-0329.1999.00128.x.
Gimeno T.E., Pias B., Lemos-Filhos J.P., Valladares F. (2009). Plasticity and stress tolerance override local adaptation in the response of Mediterranean holm oak seedlings to drought and cold. Tree Physiology 29(1): 87–98. https://doi.org/10.1093/treephys/tpn007.
Gratani L., Meneghini M., Pesoli P., Crescente M.F. (2003). Structural and functional plasticity of Quercus ilex seedlings of different provenances in Italy. Trees-Structures and Function 17(6): 515–521. https://doi.org/10.1007/s00468-003-0269-8.
Horta M., Caetano P., Medeira C., Maia I., Cravador A. (2010). Involvement of the b-cinnamomin elicitin in infection and colonisation of cork oak roots by Phytophthora cinnamomi. European Journal of Plant Pathology 127(3): 427–436. https://doi.org/10.1007/s10658-010-9609-x.
Luque J., Cohen M., Save R., Biel C., Alvarez I.F. (1999). Effects of three fungal pathogens on water relations, chlorophyll fluorescence and growth of Quercus suber L. Annals of Forest Science 56(1): 19–26. https://doi.org/10.1051/forest:19990103.
Malajczuk N., McComb A.J., Parker C.A. (1977). Infection by Phytophthora cinnamomi Rands of roots of Eucalyptus calophylla R. Br. and Eucalyptus marginata Donn.ex Sm. Australian Journal of Botany 25(5): 483–500. https://doi.org/10.1071/BT9770483.
Marçais B., Dupuis F., Desprez-Loustau M.L. (1996). Susceptibility of the Quercus rubra root system to Phytophthora cinnamomi; comparison with chestnut and other oak species. European Journal of Forest Pathology 26(3): 133–143. https://doi.org/10.1111/j.1439-0329.1996.tb00718.x.
Miller P.M. (1955). V-8 juice agar as a general-purpose medium for fungi and bacteria. Phytopathology 45(8): 461–462.
Moralejo E., García-Muñoz J.A., Descals E. (2009). Susceptibility of Iberian trees to Phytophthora ramorum and P. cinnamomi. Plant Pathology 58(2): 271–283. https://doi.org/10.1111/j.1365-3059.2008.01956.x.
Moreira A.C., Martins J.M.S. (2005). Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. Forest Pathology 35(3): 145–162. https://doi.org/10.1111/j.1439-0329.2005.00397.x.
Ogaya R., Peñuelas J. (2003). Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environmental and Experimental Botany 50(2): 137–148. https://doi.org/10.1016/S0098-8472(03)00019-4.
Podger F.D. (1972). Phytophthora cinnamomi, a cause of lethal disease in indigenous plant communities in Western Australia. Phytopathology 62(1): 972–981. https://doi.org/10.1094/Phyto-62-972.
Robin C., Desprez-Loustau M.L. (1998). Testing variability in pathogenicity of Phytophthora cinnamomi. European Journal of Plant Pathology 104(5): 465–475. https://doi.org/10.1023/A:1008649806620.
Robin C., Capron G., Desprez-Loustau M.L. (2001). Root infection by Phytophthora cinnamomi in seedlings of three oak species. Plant Pathology 50(6): 708–716. https://doi.org/10.1046/j.1365-3059.2001.00643.x.
Rodríguez Molina M.C., Torres Vila L.M., Blanco Santos A., Palo Núñez E.J., Torres Álvarez E. (2002). Viability of holm and cork oak seedlings from acorns sown in soils naturally infected with Phytophthora cinnamomi. Forest Pathology 32: 365–372. https://doi.org/10.1046/j.1439-0329.2002.00297.x.
Sánchez M.E., Caetano P., Ferraz J., Trapero A. (2002). Phytophthora disease of Quercus ilex in south-western Spain. Forest Pathology 32: 5–18. https://doi.org/10.1046/j.1439-0329.2002.00261.x.
Sánchez M.E., Andicoberry S., Trapero A. (2005). Pathogenicity of three Phytophthora spp. causing late seedling root of Quercus ilex ssp. ballota. Forest Pathology 35: 115–125. https://doi.org/10.1111/j.1439-0329.2004.00392.x.
Sánchez-Hernández M.E., Muñoz-García M., Brasier C.M., Trapero-Casas A. (2001). Identity and pathogenicity of two Phytophthora taxa associated with a new root disease of olive trees. Plant Disease 85(4): 411–416. https://doi.org/10.1094/PDIS.2001.85.4.411.
Serrano M.S., De Vita P., Carbonero M.D., Fernández F., Fernández-Rebollo P., Sánchez M.E. (2012a). Susceptibility to Phytophthora cinnamomi of the commonest morphotypes of Holm oak in southern Spain. Forest Pathology 42(4): 345–347. https://doi.org/10.1111/j.1439-0329.2011.00758.x.
Serrano M.S., Fernández-Rebollo P., De Vita P., Sánchez M.E. (2012b). Susceptibility of common herbaceous crops to Phytophthora cinnamomi and its influence on Quercus root rot in rangelands. European Journal of Plant Pathology 134(2): 409–414. https://doi.org/10.1007/s10658-012-9999-z.
Sghaier-Hammami B., Valero-Galván J., Cristina Romero-Rodríguez M.C., Navarro-Cerrillo R.M., Abdelly C., Jorrín-Novo J. (2013). Physiological and proteomics analyses of Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) responses to Phytophthora cinnamomi. Plant Physiology and Biochemistry 71: 191–202. https://doi.org/10.1016/j.plaphy.2013.06.030.
Tapias R., Fernández M., Sáenz A., Alcuña M.M., José V., Inchusa A., Moreira A.C., Cravador A. (2004). Variability of tolerance/resistance of Quercus suber L. seedlings to Phytophthora cinnamomi Rands. Evaluation of survival. Universidad de Huelva. Vol. 500. 2008. p. 237–246.
Widmer T.L., Shishkoff N., Dodge S.C. (2012). Infectivity and inoculum production of Phytophthora ramorum on roots of Eastern United States oak species. Plant Disease 96(11): 1675–1682. https://doi.org/10.1094/PDIS-12-11-1024-RE.
Total of 34 references.

