Does slope exposure affect frost ring formation in Picea obovata growing at treeline in the Southern Urals?
Gurskaya M., Moiseev P., Wilmking M. (2016). Does slope exposure affect frost ring formation in Picea obovata growing at treeline in the Southern Urals? Silva Fennica vol. 50 no. 3 article id 1560. https://doi.org/10.14214/sf.1560
Highlights
- Frost ring formation was ubiquitous from continuous forest cover to treeline, but, surprisingly, was not affected by slope exposure
- Late frosts in spring were the main cause for frost ring formation
- While mainly young trees (< 30 years) recorded frost events, at the climatically harshest site (highest elevation, northeastern exposure), frost events were recorded also in trees older than 70 years.
Abstract
Topographic complexity in mountainous ecosystems strongly influences plant growth and as such also wood formation. This wood formation can possibly be used to understand topographic variation of the main climatic drivers, e.g. by modulating frost events. Here we test the influence of different slope exposures on the spatio-temporal distribution of frost rings in Siberian spruce (Picea obovata Ledeb.) in the Southern Urals, Russia. We sampled on two opposite slopes, northeast (NE) and southwest (SW), on three elevation levels from the highest single trees to closed canopy forest and analysed frost ring occurrence and their seasonal timing. Frost ring formation at all exposure-elevation combinations was common and mainly concentrated in the early part of the growing season. The age until trees record frost rings was equally similar (until about 35 years) on both slopes and different elevational levels with the exception of the climatically harshest site, the highest elevation on the NE slope. While we could not deduce a direct, easily identifiable climatic driver for the formation of frost rings, our analysis shows high potential to disentangle the complex interplay between climate, site condition and tree growth in mountainous ecosystems.
Keywords
tree line;
topography;
frost injury;
Siberian spruce
-
Gurskaya,
Institute of Plant and Animal Ecology (IPAE), Ural Branch of Russian Academy of Sciences , 8 Marta St. 202, 620144 Ekaterinburg, Russia
E-mail
mgurskaya@yandex.ru

- Moiseev, Institute of Plant and Animal Ecology (IPAE), Ural Branch of Russian Academy of Sciences , 8 Marta St. 202, 620144 Ekaterinburg, Russia E-mail moiseev@ipae.uran.ru
- Wilmking, Institute of Botany and Landscape Ecology, Soldmanstrasse 15, Ernst-Moritz-Arndt-University Greifswald, 17487 Greifswald, Germany E-mail wilmking@uni-greifswald.de
Received 3 February 2016 Accepted 10 May 2016 Published 23 May 2016
Views 150789
Available at https://doi.org/10.14214/sf.1560 | Download PDF

Supplementary Files
1 Introduction
Topographic complexity in mountainous ecosystems influences the amount of incoming and reflected solar radiation (Rosenberg et al. 1983) and there by air and soil temperature (Rorison 1986; Fekedulegn et al. 2003), quantity and distribution of precipitation (Sternberg and Shoshany 2001; Bennie et al. 2006; Wang et al. 2011), relative humidity (Fekedulegn et al. 2003), wind regime (Barry 2008; Lavoie and Payette 1992), snow cover (Lavoie and Payette 1992), speed of snow melt (Carey and Woo 1999), evaporation dynamics (Degen et al. 1992) and drainage (Munro and Huang 1998). These factors are essentially reflected in the structure and function of mountainous ecosystems (Piggott 1975) and their compartments: soils and microbiomes (Rech et al. 2001; Carletti et al. 2009), and flora and fauna (Broza and Nevo 1996; Hennenberg and Bruelheide 2003). Studies of microclimate, growth and regeneration of woody plants growing on different topographic exposures might be a good tool to better understand the fine scale complexity of these landscapes, using the growth record laid down every year in the wood of trees and shrubs (Badano et al. 2005; Leonelli et al. 2009; Elliott and Kipfmueller 2010).
Northern and southern slopes have essential differences in their energy budget mainly in winter (up to 20–30%), and at the beginning and end of the growth period, when sun angles are low over the horizon (Golubeva 1966; Cazorzi and Dalla Fontana 1996). During summer, solar radiation income is more evenly distributed, but also then, minimum amounts of radiation are observed on north-facing slopes of the northern hemisphere (Zakharova 1959; Koerner 2012).
While direct meteorological observations are often used to measure or estimate microclimatic conditions of different exposures (Kutiel 1992; Fekedulegn et al. 2003; Bennie et al. 2008), meteorological measurements are usually short, and the use of data from high mountain weather stations is not always possible, because the stations are often located on the mountain tops. Remote sensing and digital models (e.g. GIS based) of solar radiation, temperature and precipitations are recent developments to estimate microclimatic conditions on different exposures (Lassueur et al. 2006), but also there, observational evidence is restricted to recent short time periods. In addition those models are limited in their ability to resolve short term weather fluctuations, such as extreme events (e.g. frost) (Barry 2008), which essentially impact ecosystem structure and function.
Frost injuries of woody tissue, e.g. in the tracheids of conifers, can be used to infer timing and intensity of frost events (Glerum and Farrar 1966; Gurskaya and Shiyatov 2002; Schweingruber 2007; Payette et al. 2010). These so called “Frost rings” are composed of several layers of cells containing for example: crumpled tracheids, black amorphous layers, bent xylem ray cells, crushed or collapsed cells and traumatic parenchyma layers as well as restored tracheid layers (Glerum and Farrar 1966; Stöckly and Schweingruber 1996; Gurskaya and Shiyatov 2002). They are generally indicative of a frost event during the period of wood formation. Frost rings normally occur as the result of freezing water and thus expanding ice crystals inside the lumen of the wood cells, damaging cell wall structures. Or they can form when after a frost event a sudden increase in temperature leads to high evaporative demands which cannot be met since ice or increased water tension inhibits or slows water flow up the stems. The resulting water stress can break the water column and consequently lead to the collapse of unlignified xylem cells (Stöckli 1996; Oertli 1993; Grace 1994 in Schweingruber 2007). Frost rings can clearly be identified under the microscope. Experimentally, frost ring were stimulated byfreezing temperature below –8 °C (Glerum and Farrar 1966), but studies in natural environments have shown that temperature of around 0°C can already lead to the formation of specific structures in the xylem of coniferous trees, indicative of frost (Stöckly and Schweingruber 1996; Gurskaya 2014). While thick bark and high thermal capacity of thick stems can protect cambium cells from low temperatures during frost events, younger and smaller trees with thin bark are damaged more easily (Gurskaya and Shiyatov 2006).
In this paper we address possible effects of different exposures (southwest versus northeast) on 1) spatio-temporal distribution of frost rings and 2) intensity of frost damage in the wood of Siberian spruce (Picea obovata Ledeb.), growing at the upper tree line ecotone in the Southern Ural Mountains, Russia.
2 Material and methods
2.1 Site description
The research area was located on the Iremel Massif (1582 m above sea level), one of the highest peaks in the central Southern Ural Mountains (54°32´N, 58°52´E). The compact mountain group has an oval form, extending 20 km from the northeast to the southwest. Our sampling area was between two peaks: Bolshoy and Maly Iremel, comprised of quartz sandstones and coaly slates, and separated by a 3 km flat saddle (Fig. 1). The upper part of this high mountain valley on the Iremel Massif is characterized by gentle slopes, with slope angels of not more than 3–5 degrees.
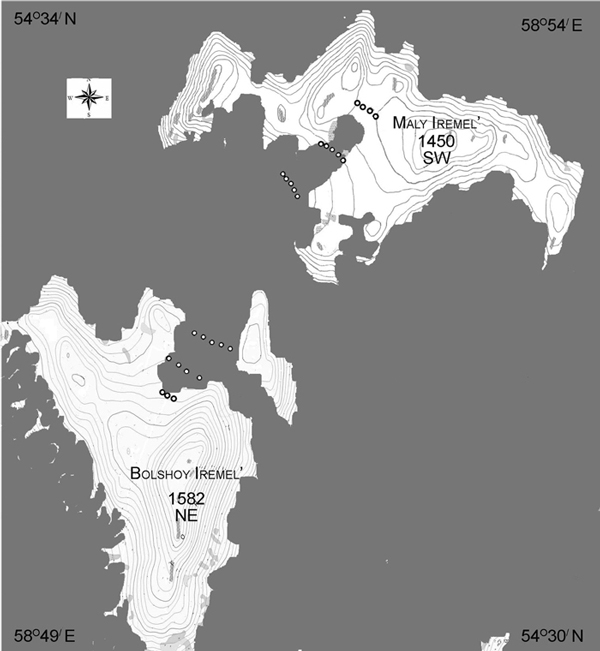
Fig. 1. Study area in the southern Ural Mountains, Russia. Northeast (NE, Bolshoy Iremel) and southwest (SW, Maly Iremel) exposures, plots (сircles) at three elevational levels per exposure from closed canopy forest to treeline were sampled. Continuous forest cover in grey.
We used data from 26 plots at the upper tree line ecotone, established between 2002–2004, each measuring 20 × 20 m. Twelve plots were located on the northeast-facing slope of Bolshoy Iremel (NE) at three elevational levels from closed canopy forest (NE3) to treeline (NE1). Fourteen plots were located on the southwest-facing slope of Maly Iremel (SW), also on three elevational levels from closed canopy forest (SW3) to treeline (SW1). Detailed descriptions of the site and sampled trees are provided in Table 1 and by Trubina (2006) and Koshkina et al. (2008).
| Table 1. General characteristics of the study sites. | ||||||
| Site name | Mt. Bolshoi Iremel (NE exposure) | Mt. Maly Iremel (SW exposure) | ||||
| NE1 | NE2 | NE3 | SW1 | SW2 | SW3 | |
| Elevation in m above sea level | 1365 | 1335 | 1290 | 1360 | 1310 | 1260 |
| Number of plots | 3 | 4 | 5 | 4 | 5 | 5 |
| Tree cover in % | 10 | 25 | 48 | 5 | 30 | 50 |
| Number of trees sampled | 18 | 260 | 261 | 11 | 94 | 139 |
| Mean tree height in m | 2.5 ± 0.2 | 5.3 ± 0.1 | 5.2 ± 0.2 | 1.7 ± 0.2 | 3.3 ± 0.2 | 5.3 ± 0.2 |
| Maximum tree height | 4.3 | 7.5 | 11.0 | 4.1 | 9.2 | 13.0 |
| Mean tree diameter at breast height in cm | 3.6 ± 0.5 | 2.4 ± 0.4 | 12.3 ± 0.6 | 3.1 ± 0.6 | 7.5 ± 0.5 | 10.4 ± 0.6 |
| Maximum tree diameter at breast height in cm | 11.0 | 31.0 | 32.0 | 11.8 | 27.4 | 36.6 |
| Mean tree age in years | 72 ± 8 | 70 ± 4 | 80 ± 3 | 38 ± 4 | 48 ± 3 | 74 ± 3 |
| Maximum tree age in years | 116 | 290 | 211 | 72 | 105 | 124 |
| NE = northeast, SW = southwest | ||||||
2.2 Climate data
We used long term daily climate data from the Taganai-gora weather station, located 115 km northeast of the study area in mountainous tundra (1114 m a.s.l., period of observation 1933–2001), supplemented by local measurements over the last 10 years (2005–2015). January mean monthly temperature at Taganai-gora is –14.5 °С, and July mean monthly temperature is +12.3 °С. The growth period (mean daily temperature > 5 °С) is 125 days on average. Precipitation ranges from 600–1300 mm year–1, with an average of 936 mm. The average amount of winter precipitation (October–March) is 243 mm, mean snow cover depth in the second half of March, is 88.5 cm.
To estimate a local environmental lapse rate, we used the temperature data observed directly on the Iremel Massif (2005–2015). Mini weather stations (HOBO, Onset Cooperation) were placed on 1360 m a.s.l. and on 1265 m a.s.l. on the southwest-facing slope. The resulting mean temperature change was 0.2 °C per 95 m and was subsequently applied to the daily temperature measurements from the Taganai-gora station. In 2008 air temperature was measured every 4 hours also on the northeast-facing slope (14.06–15.09).
We defined the beginning of the growth period as three consecutive days with mean daily temperatures above 8 °С (Rossi et al. 2008), and a day with frost, when minimum daily temperature was below 0°С. Since from Taganai-gora only average daily temperatures were available, we used the locally measured daily temperature amplitudes of cold days (< 8 °С) from May–August, divided them by two and subtracted that value from the mean daily temperature value of Taganai-gora to estimate a minimum daily temperature. Analysis of diurnal temperature amplitude measured by the HOBO data-loggers on the Iremel Massiv has shown, that in those days, when mean daily temperature dropped below 8 °С, the diurnal temperature amplitude was around 4 °С or slightly less. Thus, we defined days with frost as those days, when mean daily temperature was around 2 °С at the study site and the minimum daily temperature thus most likely dropped below 0°С.
We divided the May–August climate record in four growth periods, corresponding to the seasonal dynamics of spruce growth in the study area (Goryachev 1991). Growth periods overlapped 5 days. The first period corresponded to 1st May – 15th of June (start of earlywood formation), the second period (mainly related to the formation of the earlywood) corresponded to 10th – 30th of June. The third period (final earlywood formation and maturation) covered 25th of June to 25th of July and the fourth period (formation of latewood tracheids and final maturation) covered 20th of July to end of August. In case of several frost periods within the predefined four growth periods, we used the climate values of the last frost event in each growth period respectively for the analysis.
2.3 Data processing
All spruce trees were cored at 0.5 m above ground level in order to obtain a long record. We collected samples from 18, 260, 261 trees from NE1, NE2 and NE3, respectively and 11, 94, 139 trees from SW1, SW2 and SW3 respectively.
Each core was polished and a tooth powder was rubbed on the polished surface to increase contrast of the tree rings. The samples were measured and crossdated using TSAP–3.0 (Rinn 1996) and COFECHA (Holmes 1983). We hit the pith in 80% of the samples. When not hit, the number of missed rings was estimated by an overlay grid to calculate the correct cambial age at that height of the stem. Due to the small slope angles, most of the samples do not have a strongly pronounced eccentricity.
Frost damage was identified under a light microscope (compare Fig. 2A–C) and the relative position of the indicative structures within each tree ring was defined parallel to the growth periods defined in the climate data. We divided each growth ring in four growth periods: GP1, the beginning of annual growth, right at the zone after the latewood of the previous annual ring. Frost damage there translates into earlywood of the current ring without any intact tracheid between frost damage and ring boundary; GP2, the first half of the early wood; GP3, the second half of the early wood; and GP4, the latewood (Fig. 2). We developed frost ring chronologies based on the percentage of damaged rings in a particular year for each slope and each elevational level (Supplementary file 1).
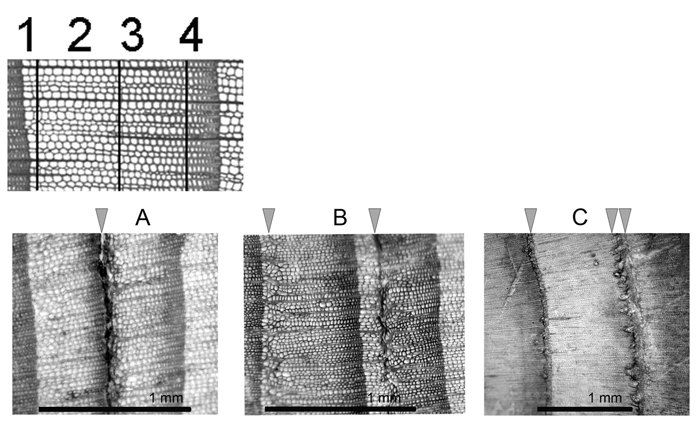
Fig. 2. Growth periods (GP) of an annual ring, growing from left to right. The position of frost damage can be used as an indicator for the seasonal driver of frost events. Frost damage in early spring would register in the beginning of earlywood (1, A, C). Late frost events can further register in the first half of the earlywood (2, B). Frost events in the second half of earlywood might be mid-season frosts (3, B). Early frost at the end of the growing season would result in a frost ring in the latewood (4, C).
We calculated the frequency of frost rings in 5-year age classes starting from the pith for every elevational level. We then defined the age, at which trees lose sensitivity to frost because of increasing size and bark thickness, using a 90% threshold, i.e. the age below which 90% of all frost rings at a certain elevation-exposure combination were recorded. In the following analyses, we then only used trees younger than our cut-off age and we only analyzed the record, where data from at least two elevational levels for both exposures were available (1920–2004). To estimate differences between the two exposures and growth periods, we used a t-test for temperature analysis and a Kruskal-Wallis H-test and a median test for frost ring data. Percentage of damaged rings per year was used to test for a correlation between frost damages and mean daily temperature, estimated by linear regression with significance at a p-value < 0.05.
3 Results
3.1 Microclimatic differences between slopes.
During the growth period of 2008, minimum June air temperatures were on average 1.2 °C higher at NE1 compared to SW1 and maximum differences reached 6.5 °C in June. No significant differences between minimal temperature values of the two exposures existed from the second part of July–August onwards. Meanwhile, minimum air temperatures at NE3 and SW3 remained very similar during the whole growth period. Observed differences up to 1 °C on particular days were not statistically significant. No significant differences between maximum air temperatures on the two slopes and on both elevations were revealed (Fig. 3).
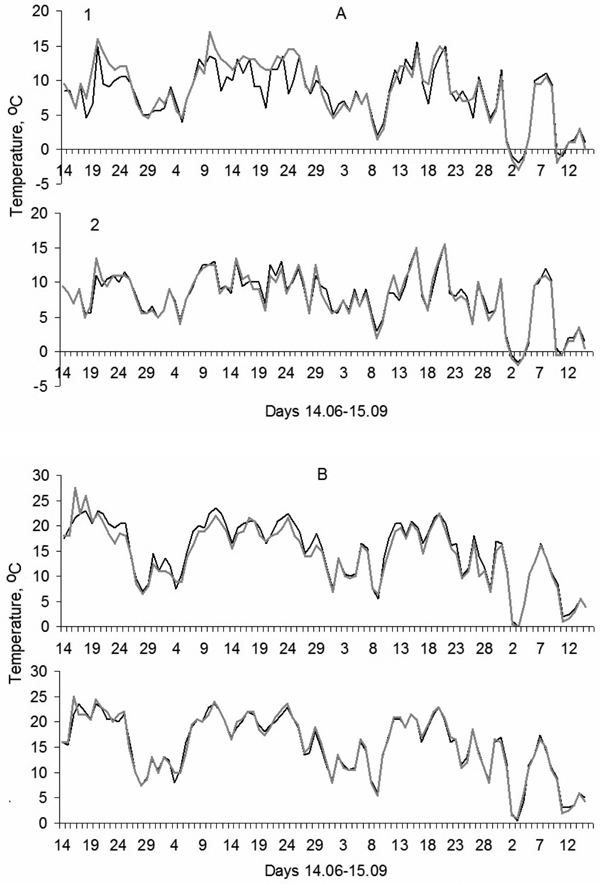
Fig. 3. Daily minimum (A) and maximum (B) temperatures in 2008 at the highest elevation at treeline (1) and in continuous forest (2) on the southwest (black) and northeast (grey) -facing slopes.
3.2 Frost ring chronologies
The common time period, for which data on frost ring occurrence for all six exposure-elevation combinations was available, was 1941–2004. From 1920 to 1940, data was available for the middle and lower levels on both exposures. From 1900 to 1919, data on frost rings was available only from the lower elevations on both exposures and thus this period was not used for further analysis (Suppl. file 1). The number of trees contributing to the frost ring chronologies was generally relatively low at the beginning and end of the record. From the middle and lower elevational levels usually more than 20 trees contributed to the chronologies, while the highest elevational level had a replication below 20 (Fig. 4).
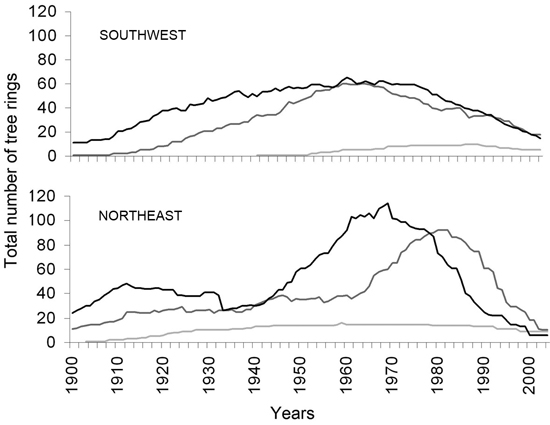
Fig. 4. Sample depth of trees contributing to our analysis by exposure. Lowest elevational level with continuous forest cover (dark line), middle elevational level (middle gray line), and highest elevational level at treeline (light lower gray line). Sample size is largest in the middle of the record and decreases sharply at the end, sample size is lowest at highest elevations due to lower tree density in our area based plot design.
Frost ring numbers in a single tree differed between 1 and 14. Overall, they occurred more often in trees from the upper elevational levels, with mean values of seven frost rings per tree. At the middle and lower elevational levels on average three frost rings per tree were present in the samples. However, at least one tree at every elevational level showed very high numbers of frost rings. There were no distinct differences in frost ring number between the two slopes (Fig. 5).
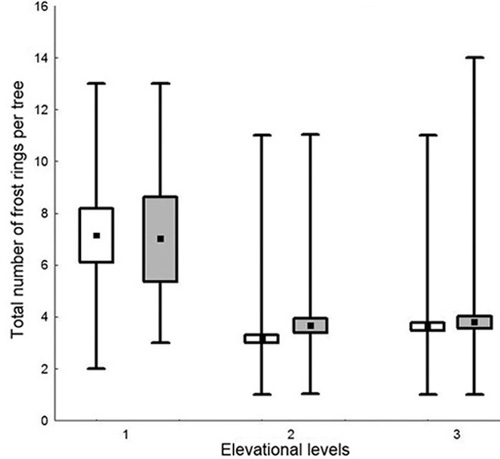
Fig. 5. Frost ring number per tree on the northeast (white boxes) and southwest (grey boxes) exposures. Mean value (black square), standard error (rectangle box), min-max values (whiskers). On average more frost rings occurred higher up the slope at elevational level 1 (treeline), compared to mid slope (2) and continuous forest cover (3).
3.3 Frost sensitivity versus tree age
In general, differences in frost sensitivity were low between different exposures and elevational levels. At five out of six exposure-elevation combinations, we found 90% of all frost rings formed in trees with cambial age younger than 40 years. The only exception was the highest elevational level on the NE-facing slope, where 90% of all frost rings formed in trees with cambial age younger than 75 years. However, in general, the maximal cambial age of tree sensitivity to frost was higher on the NE-facing slope (Fig. 6).
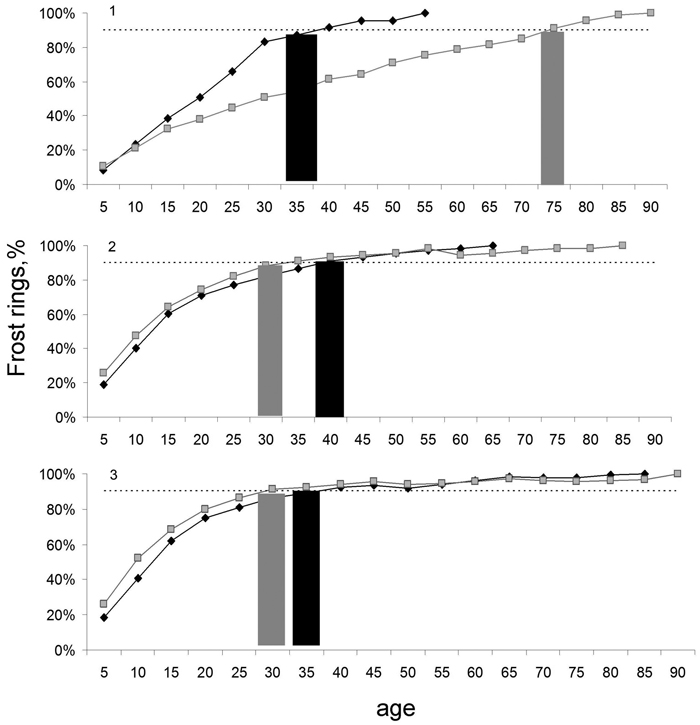
Fig. 6. Effect of slope exposure and tree age on frost sensitivity of spruce. Cumulative amount of frost rings at northeast (grey line with squares) and southwest (black line with diamonds) exposures on three elevational levels (1, 2, 3). Frost-sensitive tree age (northeast grey bar, southwest black bar) defined as the age below which 90% of all frost rings (horizontal line) at that elevation-exposure combination occurred. Most frost rings occurred at an age below 30 years with the exception of the climatically harshest site, the highest elevation on the northeast exposure.
3.4 Spatio-temporal signature of frost damage
Frost effects were recorded in nearly every year with the exception of 1917, 1928, 1929 (data only from two lower elevations), 1940, 1971, 1976, 1977, 1990, 1991, 1996, 2000, 2002, 2003, 2004 (Suppl. file 1). Usually both exposures were affected and most often all elevational levels, but to a varying degree and without a clear pattern of which elevational level registered most frost effects. When frost rings formed on only one and not the other exposure, they formed mainly on the NE exposure and this pattern was true for both earlywood and latewood (Supplementary file 2).
In general, frost rings formed mainly in the earlywood. We found most frost effects (between 2 and 8% of all rings) in the earliest part of the growing season (GP1), followed by the second part of the growing season (GP2) with 2–4% frost rings. GP3 and GP4 showed the least amount of frost damage with generally less than 2% frost rings (Fig. 7). There were no significant differences in the relative amount of frost rings between different elevational levels of the same exposure (results not shown). In the early part of the growing period, in GP1, NE-facing slopes generally recorded more frost damage than SW-facing slopes, however, significantly so only at the middle elevational level. In GP2, no significant differences in the amount of frost damage between exposures existed. The pattern in GP3 was similar to GP4, with no significant differences at the highest elevational level, but significantly more frost damage on the SW-facing slope at the middle and lower elevational level (Fig. 7).
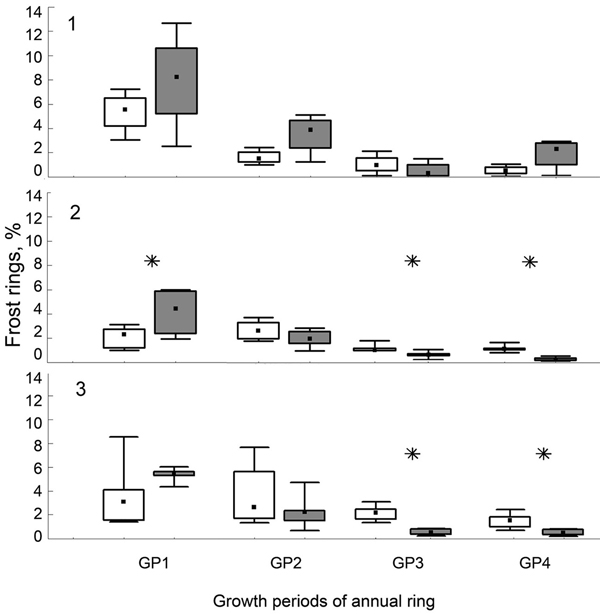
Fig. 7. Percentage of tree rings showing frost events in a particular year from frost sensitive trees (see Fig. 6) on the northeast (white box) and southwest (grey box) exposures, grouped by timing of the frost events. GP (growth periods, 1: early spring to 4: late summer), Median (black square), 25–75% (rectangle box), extreme values (segment), elevational levels (1, treeline; 2, mid elevational slope; 3, continuous forest cover), significant differences between slopes marked by asterisk.
3.5 Temperature – frost ring relationships
In the first half of the growing season (GP1 and GP2), frost damage was recorded with minimum daily temperatures below 0°C (Table 2). While a general trend of colder days and more frost damage seemed to be apparent in some exposure-elevation combinations, this result was not ubiquitous and generally also not significant (exception lowest elevational level of the NE-facing slope in GP1) (Fig. 8).
| Table 2. Years of frost ring formation on both slopes (left), or only on one slope (middle part for northeast (NE) and right for southwest (SW) slope). Mean daily temperature from the Taganai-gora weather station. View in new window/tab. |
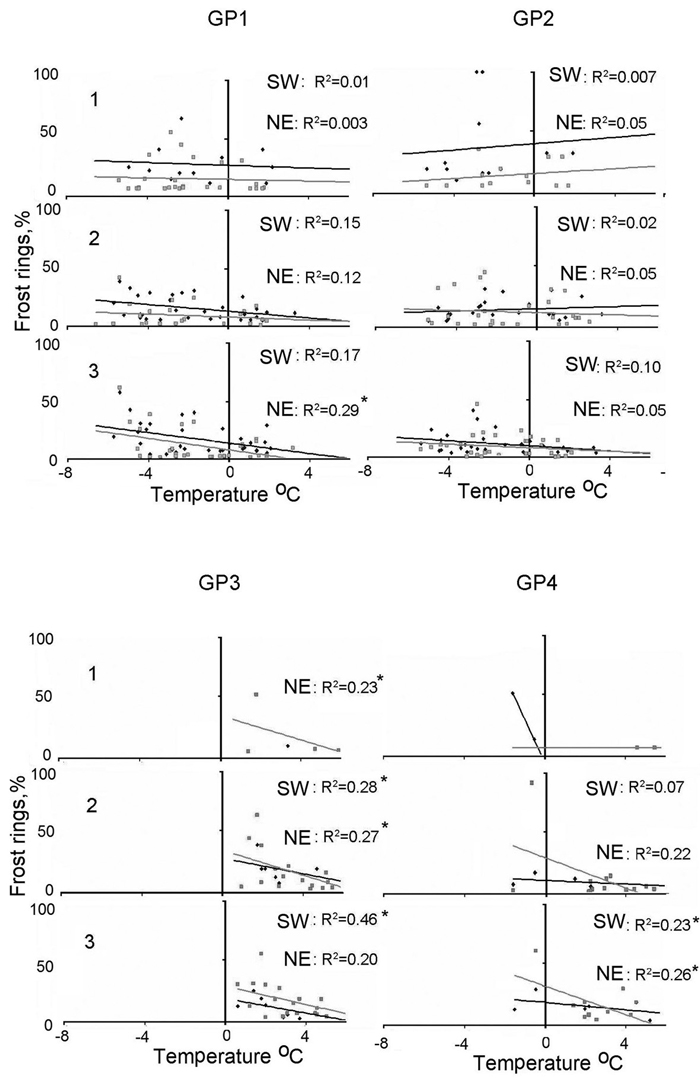
Fig. 8. Correlations between frost ring formation and mean daily temperature at the three elevational levels (1–3), two exposures (NE = northeast, gray square, SW = southwest, black rhomb) are most often not significant. Linear regression line: northeast (grey line), southwest (black line), significant correlations p-value < 0.05 (*). Please note that a mean daily temperature of +2 °C is most likely related to a frost event, since the daily temperature amplitude in those times of the year is about 4 °C. GP1–4 = growth periods from early spring to late summer.
In the second half of the growth period (GP3 and GP4), frost damage was recorded with minimum temperatures well above 0°C. In GP3 all relationships with sufficient samples (exception here highest elevational level on both exposures) between minimum daily temperature and frost damage were significantly correlated, with colder temperatures leading to more frost damage. In GP4 significant relationships between daily minimum temperature and frost rings occurred only at the lowest elevational level of the NE-facing slope. In most cases, the correlation coefficients increased towards lower elevation (Fig. 8). In general there were no large differences in the climate-frost ring relationships between the two exposures.
4 Discussion
Frost rings formed often in Picea obovata growing at the upper tree-line ecotone in the Southern Urals. Actually, it was possible at every elevational level to find a tree with many frost rings (around 12–14) and with only one frost ring, highlighting strong individual differences. On average though, frost rings formed more often on the highest elevational level, indicating that elevation seems to have more influence on the frequency of frost ring formation than exposure.
Coniferous trees usually record frost rings at ages below ca. 30 years (Day and Peace 1937; Glock 1951; Glerum and Farrar 1966; Payette et al. 2010), because thicker barks and more heat capacity of larger stems make older and larger trees less susceptible to frost damage (Gurskaya and Shiyatov 2006). Our results are generally in line with these findings, but deviate significantly at one location, the highest elevation on the NE exposure. Here, trees record frost damage until about 75 years. The most likely explanation for the differences might be the locally most extreme climatic conditions, where the highest elevation at NE exposure might be most prone to frost events. But keep in mind that results at the highest elevational level are to some extend compromised by the relatively small sample size of around ten trees. However, further frost ring based evidence that the NE exposure is climatically extremer than the SW exposure stems from the fact that if frost rings are registered on only one exposure, it is most often the NE exposure. In those cases lower elevational levels can be affected as well, e.g. in 1972, 1973, 1980, and 1997, showing that the more extreme climatic conditions are not restricted to the highest elevational zone alone.
When comparing differences between slopes with respect to timing of the registered frost ring, it becomes relatively clear that 1) no statistically significant distinctions between differing exposures exist, 2) the earliest cells of an annual ring are most at risk for frost injury, and 3) mostly so on the NE exposure. However, hardly any significant relationships exist between the occurrence of frost rings in the early part of the growth ring and daily mean temperatures. This might be related to the variability of the timing of snowmelt (Vaganov et al. 1999; Kirdyanov et al. 2003) and/or to the beginning of the growing season along elevational gradients and differing exposures (Rossi et al. 2011), or simply to the fact that our data might not spatially resolve strong temperature fluctuations, which potentially could also lead to frost ring formation.
On the other hand, significant relationships do exist between the occurrence of frost rings and daily minimum temperatures at the end of the growing season (GP3 and GP4), with colder temperatures leading to more frost injuries. At the end of the growth period, trees on the SW exposure were more affected by frost injuries, most likely due to higher mean diurnal range of temperatures during early autumn frosts (Stökly and Schweingruber 1996; Tang and Fang 2006) on the SW-facing slope (Desplanque et al. 1999; Rossi et al. 2007; Leonelli et al. 2009). However, several studies show that temperature does not seem to influence the termination of tree-ring growth (Deslauriers et al. 2008; Moser et al. 2010). Instead tracheid maturation and growth cessation is rather controlled by the photoperiod (Jackson 2008; Way and Montgomery 2014). Therefore the general cause of more late frost damage on the SW exposure is hardly resolvable by our data.
The prevalence of frost damage on the NE exposure in some years is probably a consequence of up to 20–30% less solar income and 8–20 days of snowmelt delay (Cazorzi and Dalla Fontana 1996) compared with the SW exposure, resulting in more and stronger frost events. Conditions within a specific year leading to frost ring formation on the NE-facing slope are sometimes subsequently recorded in different stages (mainly GP1 and GP2) of tree ring formation in different trees. This hints at different timing regarding the onset of xylogenesis in different trees. It would be good to analyze frost ring data of individual trees using spatially explicit data about snow and soil melt, and, thus possibly, correct the dates of the beginning of the growing season of these years for individual trees accordingly. Unfortunately, spatially highly resolved snow data are not available for our study site, and thus this hypothesis a waits further testing.
Noteworthy are also the absolute values of temperatures leading to the formation of frost rings. While in frost rings during earlywood formation, the minimum temperatures are in the range of 0°C (mean of 2 °C) or below, concurrent with other studies (e.g. Stöckly and Schweingruber 1996; Gurskaya and Shiyatov 2002; Panayotov and Yurukov 2007; Gurskaya 2014), the temperatures leading to formation of frost injury in latewood are most likely above 0°C when we take the general diurnal temperature range into account. The sensitivity of mother xylems cells and expanded and maturated tracheids to low temperature around 0°C increases during the growth season. During the second part of growth period (GP3 and GP4) frost hardening of trees is usually reduced (Greer et al. 2000). Probably, lower temperatures during this period could influence frost ring formation (especially the type of frost rings without an amorphous layer of dead tracheids, see e.g. Fig. 2C). To gain a better understanding into these processes, we thus highly recommend using sites where fine scale, topographically resolved, high resolution climate records are available.
An additional difficulty when working with this fine resolution data is the necessity to estimate the onset of the growing season for each individual exposure-elevation combination. For example it could be the case that trees at the lower elevational level have started to form tracheids, because of an earlier onset of the growing period there, when a frost hits. This event would then only be registered at the lowest level. On the other hand, a later frost event might register in GP1 at the highest level and in GP2 at the lower level, simply because the lower level might have formed the first tracheids some days before, while the higher level has just started xylogenesis. Differences in the beginning of the growing season along elevational levels have been reported frequently in the literature (e.g. Villalba et al. 1994; Oberhuber and Kofler 2000; Vaganov et al. 2006; Moser et al. 2008; Koerner 2012).The resulting analytical difficulties along elevational gradients might be applicable to differing exposures as well with the NE-facing slope in our case most likely behaving similar to the higher elevational levels. To solve this, additional investigations in the Southern Urals are required.
It is also noteworthy that the beginning of the 21st century, at least the first four years, from which we have a record, has been a period with very little frost injury recorded and the only period in the analysis showing three consecutive years without any frost injury. The single year, where frost injury was recorded, it was only recorded at the highest elevation of the SW exposure. While this might be partly attributed to the lower sample size at the end of the record, it might also be the result of exceptionally warm temperatures in the area during that period. However, the Taganai-gora record only covers the period until 2001 and so the cause for no frost injuries remains unclear and speculative.
5 Conclusions
Frost ring formation in spruce, growing at the upper tree line in the South Ural Mountains on two opposed facing slopes (southwest- and northeast-facing slopes) is common. Mainly, our analysis showed frost ring formation in the early part of the growing season, irrespective of exposure and elevation. The age until trees record frost rings is equally similar (until about 35 years) on both slopes and different elevational levels with the exception of the climatically harshest site, the highest elevation on the NE slope. We could not deduce a direct, easily identifiable climatic driver for the formation of frost rings, which might be due to the quality of the climate record and micro-climatic variations. Some differences we observed were related to the timing of the frost events, with different trees on the same exposure-elevation combination recording the same event during different phases of their xylogenesis. This points to a complex interplay between climate, site condition and individual tree and makes interferences about climatic drivers more complicated. It is therefore necessary to continue to develop the methods and analytical tools related to anatomical structures in tree rings and their ecological interpretation. These methods could be successfully used to reconstruct extreme weather events in the past, also in remote mountain areas, where weather observations and records are sparse.
Acknowledgements
This work was done with partial financial support of the Russian foundation of basic research (RFBR) No 14-04-91356 and 15-04-04933, program UD RAN No 15-2-4-22 and Era.net.RUS project TREELINE STProject 207. The authors thank Dr. Tobias Scharnweber for proofreading the MS.
References
Badano E.I., Cavieres L.A., Molina-Montenegro M.A., Quiroz C.L. (2005). Slope exposure influences plant association patterns in the Mediterranean matorral of Central Chile. Journal of Arid Environments 62(1): 93–108. http://dx.doi.org/10.1016/j.jaridenv.2004.10.012.
Barry R.G. (2008). Mountain weather and climate. Cambridge University Press, Cambridge, UK.
Bennie J., Hill M.O., Baxter R., Huntley B. (2006). Influence of slope and exposure on long-term vegetation change in British chalk grasslands. Journal of Ecology 94(2): 355– 368. http://dx.doi.org/10.1111/j.1365-2745.2006.01104.x.
Bennie J., Huntley B., Wiltshire A., Hill M.O., Baxter R. (2008). Slope, exposure and climate: spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecological modelling 216(1): 47–59. http://dx.doi.org/10.1016/j.ecolmodel.2008.04.010.
Broza M., Nevo E. (1996). Differentiation of the snail community on the north- and south-facing slopes of Lower Nahal Oren (Mount Carmel, Israel). Israel Journal of Zoology 42(4): 411–424.
Carey S.K., Woo M.K. (1999). Hydrology of two slopes in subarctic Yukon, Canada. Hydrological Processes 13(16): 2549–2562. http://dx.doi.org/10.1002/(SICI)1099-1085(199911)13:16<2549::AID-HYP938>3.0.CO;2-H.
Carletti P., Vendramin E., Pizzeghello D., Concheri G., ZanellaA., Nardi S.,Squartini A. (2009).Soil humic compounds and microbial communities in six spruce forests as function of parent material, slope aspect and stand age. Plant and Soil 315(1–2): 47–65. http://dx.doi.org/10.1007/s11104-008-9732-z.
Cazorzi F., Dalla Fontana G. (1996). Snowmelt modelling by combining air temperature and a distributed radiation index. Journal of Hydrology 181(1–4): 169–87. http://dx.doi.org/10.1016/0022-1694(95)02913-3.
Day W.R., Peace T.R. (1937). The influence of certain accessory factors on frost injury to forest trees. Forestry 11: 3–29.
Degen A.A., Leeper A., Shachak M. (1992). The effect of slope direction and population-density on water influx in a desert snail, Trochoidea Seetzenii. Functional Ecology 6(2): 160–166. http://dx.doi.org/10.2307/2389750.
Deslauriers А., Rossi S., Anfodillo T., Saracino A. (2007). Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiology 28(6): 863–871. http://dx.doi.org/10.1093/treephys/28.6.863.
Desplanque C., Rolland C., Schweingruber F.H. (1999). Influence of species and abiotic factors on extreme tree ring modulation: Picea abies and Abies alba in Tarentaise and Maurienne (French Alps). Trees 13: 218–227. http://dx.doi.org/10.1007/s004680050236.
Elliott G.P., Kipfmueller K.E. (2010). Multi-scale influences of slope exposure and spatial pattern on ecotonal dynamics at upper treeline in the Southern Rocky Mountains, USA. Arctic, Antarctic, and Alpine Research 42(1): 45–56. http://dx.doi.org/10.1657/1938-4246-42.1.45.
Fekedulegn D., Hicks Jr.R.R., Colbert J.J. (2003). Influence of topographic exposure, precipitation and drought on radial growth of four major tree species in an Appalachian watershed. Forest Ecology and Management 177(1–3): 409–425. http://dx.doi.org/10.1016/S0378-1127(02)00446-2.
Fritts H.C. (1976). Tree rings and climate. Academic Press, London, New York, San Francisco.
Glerum C., Farrar J. (1966). Frost ring formation in the stems of some coniferous species. Canadian Journal of Botany 44(7): 879–886. http://dx.doi.org/10.1139/b66-103.
Greer D.H., Robinson L.A., Hall J.A., Klages K, Donnison H. (2000). Frost hardening of Pinus radiata seedlings: effects of temperature on relative growth rate, carbon balance and carbohydrate concentration. Tree Physiology 20(2): 107–114. http://dx.doi.org/10.1093/treephys/20.2.107.
Glock W.S. (1923). Cambial frost injuries and multiple growth layers at Lubbokc, Texas. Ecology 32(1): 28–36. http://dx.doi.org/10.2307/1930970.
Golubeva T.A. (1966). About radiation balance of slightly slopes during the vegetation period. In: Proceedings of main geophysical observatory. Hydrometeoizdat, Leningrad, Microclimatology 190: 32–40. [In Russian].
Golzberg I.A. (1967). Microclimate of USSR. Hydrometeoizdat, Leningrad. 274 p. [In Russian].
Goryachev V.M. (1991). Seasonal growth and development of woody plants in fir-spruce forests. In: Ecological features and regeneration of dark coniferous taiga of Middle Ural Mountains. Ural Branch of Academy of Sciences of the USSR, Sverdlovsk. p. 78–100. [In Russian].
Gurskaya M.A. (2014). Temperature conditions of the formation of frost damages in conifer trees in the high latitudes of Western Siberia. Biology Bulletin 41(2): 187–196. http://dx.doi.org/10.1134/S1062359014020022.
Gurskaya M.A., Shiyatov S.G. (2002). Formation of two xylem frost injuries in one annual ring in Siberian spruce under conditions of Western Siberian forest-tundra. Russian Journal of Ecology 33(2): 83–90. http://dx.doi.org/10.1023/A:1014442705845.
Gurskaya M.A., Shiyatov S.G. (2006). Distribution of frost injuries in the wood of conifers. Russian Journal of Ecology 37(1): 7–12. http://dx.doi.org/10.1134/S1067413606010024.
Hennenberg K.J., Bruelheide H. (2003). Ecological investigations on the northern distribution range of Hippocrepis comosa L. in Germany. Plant Ecology 166(2): 167–188. http://dx.doi.org/10.1023/A:1023280109225.
Holmes R.L. (1983). Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin 43: 69–78.
Jackson S.D. (2008). Plant responses to photoperiod. New Phytolologist 181(3): 517–531. http://dx.doi.org/10.1111/j.1469-8137.2008.02681.x.
Kern Z., Popa I. (2008). Changes of frost damage and treeline advance for swiss Stone Pine in the Calimani Mts. (Eastern Carpathians, Romania). Acta Silvatica et Lignaria Hungarica 4: 39–48.
Kirdyanov A., Hughes H., Vaganov E., Schweingruber F., Silkin P. (2003). The importance of early summer temperature and date of snow melt for tree growth in Siberian Subarctic. Trees 17(1): 61–69. http://dx.doi.org/10.1007/s00468-002-0209-z.
Koerner C. (2012). Alpine treelines. Functional ecology of the global high elevation tree limits.Springer, Basel. 220 p. http://dx.doi.org/10.1007/978-3-0348-0396-0.
Koshkina N.B., Moiseev P.A., Goryaeva A.V. (2008). Reproduction of the Siberian spruce in the timberline ecotone of the Iremel Massif. Russian Journal of Ecology 39(2): 83–91. http://dx.doi.org/10.1134/S1067413608020021.
Krumm F., Kulakowski D., Spiecker H., Duc Ph., Bebi P. (2011). Stand development of Norway spruce dominated subalpine forests of the Swiss Alps. Forest Ecology and Management 262(4): 620–628. http://dx.doi.org/10.1016/j.foreco.2011.04.030.
Kutiel P. (1992). Slope aspect effect on soil and vegetation in a Mediterranean ecosystem. Israel journal of botany 41(4–6): 243–250.
Lassueur T., Joost S., Randin C. (2006). Very high resolution digital elevation models: do they improve models of plant species distribution? Ecological Modelling 198(1–2): 139–153. http://dx.doi.org/10.1016/j.ecolmodel.2006.04.004.
Lavoie C., Payette S. (1992). Black spruce growth forms as a record of a changing winter environment at treeline, Quebec, Canada. Arctic and Alpine Research 24(1): 40–49. http://dx.doi.org/10.2307/1551318.
Leonelli G., Pelfini M., Battipaglia G., Cherubini P. (2009). Site-aspect influence on climate sensitivity over time of a high-altitude Pinus cembra tree-ring network. Climatic Change 96(1): 185–201. http://dx.doi.org/10.1007/s10584-009-9574-6.
Lough J.M., Holmes R.L. (1994). Dendrochronology program library – users manual. Laboratory of Tree-Ring Research, University of Arizona, Tucson, Arizona, USA.
Moiseev P.A., van der Meer M., Rigling A., Shevchenko I.G. (2004). Effect of climatic changes on the formation of Siberian spruce generations in subgoltsy tree stands of the Southern Urals. Russian Journal of Ecology 35(3): 135–143. http://dx.doi.org/10.1023/B:RUSE.0000025962.01684.4f.
Moser L., Fonti P., BüntgenU., Esper J., Luterbacher J., Franzen J., Frank D. (2010). Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiology 30(2): 225–233. http://dx.doi.org/10.1093/treephys/tpp108.
Munro D.S., Huang L.J. (1997). Rainfall, evaporation and runoff responses to hill slope exposure in the Shenchong Basin. Catena 29(2): 131–144. http://dx.doi.org/10.1016/S0341-8162(96)00051-3.
Oberhuber W., Kofler W. (2000). Topographic influences on radial growth of Scots pine (Pinus sylvestris L.) at small spatial scales. Plant Ecology 146(2): 231–240. http://dx.doi.org/10.1023/A:1009827628125.
Panayotov M.P., Yurukov S.I. (2007). Tree ring chronology of Pinus peuce from the Pirin Mts and the possibilities to use it for climate analysis. Phytologia Balcanica 13(3): 313–320.
Payette S., Delwaide A., Simard M. (2010). Frost-ring chronologies as dendroclimatic proxies of boreal environments. Geophysical Research Letter 37(2). http://dx.doi.org/10.1029/2009gl041849.
Rech J.A., Reeves R.W., Hendricks D.M. (2001). The influence of slope aspect on soil weathering processes in the Springerville volcanic field, Arizona. Catena 43(1): 49–62. http://dx.doi.org/10.1016/S0341-8162(00)00118-1.
Rinn F. (1996). TSAP: time series analysis and presentation. Reference manual, version 3.0. Heidelberg. 262 p.
Rorison I.H., Sutton F., Hunt R. (1986). Local climate, topography and plant growth in Lathkill Dale NNR. I. A twelve-year summary of solar radiation and temperature. Plant, Cell and Environment 9: 49–56.
Rosenberg N.J., Blad B.L., Verma S.B. (1983). Microclimate: the biological environment. Wiley, New York, NY.
Rossi S., Deslauriers A., Anfodillo T., Carraro V. (2007). Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152(1): 1–12. http://dx.doi.org/10.1007/s00442-006-0625-7.
Rossi S., Deslauriers A., GričarJ., Seo J-W., Rathgeber C.BK., Anfodillo T., Morin H., Levanic T., Oven P., JalkanenR. (2008). Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography 17(6): 696–707. http://dx.doi.org/10.1111/j.1466-8238.2008.00417.x.
Rossi S., Morin H., Deslauriers A. (2011). Multi-scale influence of snowmelt on xylogenesis of black spruce. Arctic, Antarctic, and Alpine Research 43(3): 457–464. http://dx.doi.org/10.1657/1938-4246-43.3.457.
Schweingruber F.H. (2007). Wood structure and environment. Springer, Berlin-Heidelberg. 279 p.
Shiyatov S.G. (1986). Dendrochronology at the upper treeline in the Ural Mountains. Nauka, Moscow. 136 p. [In Russian].
Speer J.H. (2010). Fundamentals of tree ring research. University of Arizona Press. 333 p.
Sternberg M., Shoshany M. (2001). Influence of slope aspect on Mediterranean woody formations: comparison of a semiarid and an arid site in Israel. Ecological Research 16(2): 335– 345. http://dx.doi.org/10.1046/j.1440-1703.2001.00393.x.
Stöckli V.B., Schweingruber F.H. (1996). Tree rings as indicators of ecological processes: the influence of competition, frost, and water stress on tree growth, size and survival. Basel. 90 p.
Tang Z., Fang J. (2006). Temperature variation along the northern and southern slopes of Mt. Taibai, China. Agricultural and Forest Meteorology 139(3–4): 200–207. http://dx.doi.org/10.1016/j.agrformet.2006.07.001.
Trubina M.R. (2006). Distribution of plants differing in attitude toward thermal conditions in communities of the timberline ecotone on mount Iremel, the Southern Urals. Russian Journal of Ecology 37(5): 306–315. http://dx.doi.org/10.1134/S1067413606050031.
Vaganov E.A., Hughes M.K., Kirdyanov A.V., Schweingruber F.H., Silkin P.P. (1999). Influence of snowfall and melt timing on tree growth in subarctic Eurasia. Nature 400: 149–151. http://dx.doi.org/10.1038/22087.
Vaganov E.A., Hughes M.K., Shashkin A.W. (2006). Growth dynamics of conifer tree rings: images of past and future environments. Ecological Studies 183. Springer. 354 p.
Villalba R., Veblen T.T., Ogden J. (1994). Climatic influences on tree growth of subalpine trees in the Colorado Front Range. Ecology 75(5): 1450–1462. http://dx.doi.org/10.2307/1937468.
Wang L., Wei S.P., Hortonand R., Shao M. (2011). Effects of vegetation and slope aspect on water budget in the hill and gully region of the Loess Plateau of China. Catena 87(1): 90–91. http://dx.doi.org/10.1016/j.catena.2011.05.010.
Way D.A., Montgomery R.A. (2014). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant, Cell and Environment 38(9): 1725–1736. http://dx.doi.org/10.1111/pce.12431.
Zakharova A.F. (1959). Radiation regime of northern and southern slopes against geographical latitude. Academic proceedings of Leningrad University, Series of geographical science 13(269): 24–49. [In Russian].
Total of 63 references.

