Back to forests in pre-Saharan Morocco? When prickly pear cultivation and traditional agropastoralism reduction promote argan tree regeneration
Genin M., Alifriqui M., Fakhech A., Hafidi M., Ouahmane L., Genin D. (2017). Back to forests in pre-Saharan Morocco? When prickly pear cultivation and traditional agropastoralism reduction promote argan tree regeneration. Silva Fennica vol. 51 no. 1B article id 1618. https://doi.org/10.14214/sf.1618
Highlights
- There was a significant positive relationship between the age of implanted prickly pear orchards and natural argan tree regeneration
- This relationship is mainly associated with interconnected changes in traditional land uses and the activation of facilitation factors such as an enhancement of the soil’s organic matter and nurse plant phenomena
- This example constitutes a remarkable alternative model for thinking about agricultural development while combating desertification.
Abstract
In the southwestern pre-Saharan arid zone of Morocco, the endemic argan forest (Argania spinosa) had been almost completely destroyed in the 1960s due to intensive coal mining and mixed cereal-livestock farming. These activities turned out to be unviable and a massive rural exodus occurred in the 1970s. Local populations started to develop maintenance-free prickly pear (Opuntia ficus-indica) cultivation at large scale in order to keep their land ownership rights, while reducing their traditional agropastoral activity. We conducted a survey in order to characterize the relationships between the age of prickly pear orchards and argan tree regeneration. We also explored facilitating factors, such as soil organic matter and mycorrhiza. Results showed a high positive correlation (r2 = 0.75, p < 0.001) between the age of prickly pear orchards and argan tree resprouts, but with differences depending on a continentality gradient. The soil organic matter content also showed highly significant differences (p < 0.001) depending on the age of the prickly pear plantation, while spora density did not show such differences. The recent high economic value attributed to prickly pear fruits, and to both argan and prickly pear seed oil, has given farmers the opportunity to develop a lucrative agricultural activity, while promoting the recovery of native vegetation. This situation constitutes a remarkable example of speculative agricultural development in a very harsh environment, in phase with ecological priorities for combating desertification. It could represent an alternative to the externally-generated projects sustained by high levels of public funding, with ecological, economic and social impacts which are sometimes questionable.
Keywords
arid zones;
Argania spinosa;
agroforestry parklands;
desertification;
facilitation factors;
land use changes;
Opuntia ficus-indica
- Genin, Institut de Recherche pour le Développement (IRD) & Aix-Marseille Université, Laboratoire Population, Environnement, Développement, UMR151 AMU-IRD, Marseille, France E-mail miguel.genin@gmail.com
- Alifriqui, Cadi Ayyad University (UCAM), Laboratoire d’Ecologie et Environnement (CNRST, URAC 32), Faculté des Sciences Semlalia, Marrakech, Morocco E-mail alifriqui@gmail.com
- Fakhech, Cadi Ayyad University (UCAM), Laboratoire d’Ecologie et Environnement (CNRST, URAC 32), Faculté des Sciences Semlalia, Marrakech, Morocco E-mail abdessamad.fakhech@edu.uca.ac.ma
- Hafidi, Cadi Ayyad University (UCAM), Laboratoire d’Ecologie et Environnement (CNRST, URAC 32), Faculté des Sciences Semlalia, Marrakech, Morocco E-mail hafidi.ucam@gmail.com
- Ouahmane, Cadi Ayyad University (UCAM), Laboratoire d’Ecologie et Environnement (CNRST, URAC 32), Faculté des Sciences Semlalia, Marrakech, Morocco E-mail l.ouahmane@gmail.com
-
Genin,
Institut de Recherche pour le Développement (IRD) & Aix-Marseille Université, Laboratoire Population, Environnement, Développement, UMR151 AMU-IRD, Marseille, France
E-mail
didier.genin@univ-amu.fr

Received 20 April 2016 Accepted 26 September 2016 Published 27 February 2017
Views 291864
Available at https://doi.org/10.14214/sf.1618 | Download PDF

1 Introduction
Desertification is classically considered as a major oncoming threat which the Earth will have to deal with. The causes of desertification are still the subject of debate, but it can be considered as resulting from the combined actions of global change and human activities (Xu et al. 2014). Agricultural practices – particularly plowing and those involving the removal of soil, and clearing of natural vegetation – are often presented as major desertification factors, along with pastoral pressure. However, the processes involved are of considerable complexity, which can sometimes lead to misunderstandings of observed phenomena. For example, the generalized and irreversible advance of the Sahara desert in the 1970s was presented as resulting from misuses of land by local populations, but in fact, it has been shown to have been primarily due to conjectural abiotic factors, and to be reversible (Tucker et al. 1991). Such alternative observations led Mace (1991) to assert that: “sometimes we are so sure of something that we don’t need to see the evidence. That Africa’s rangelands are being reduced to desert due to generalized overgrazing by domestic livestock is received wisdom, but such a view may be seriously flawed ” (p. 280). However, there is localized evidence of severe human-induced land degradation in arid zones, which requires suitable measures to be adopted in order to combat desertification processes, particularly on the fringes of the major desert areas. Ligneous material, shrubs and trees, have received particular attention, since as perennial plants, they can constitute an ecosystem stabilization factor by enhancing ecosystem functioning (soil organic matter enrichment, symbiotic relationships, carbon sink role, etc.) (Zhao et al. 2007; Takimoto et al. 2008), producing biomass for local livelihoods (Adhikari et al. 2004), and limiting wind and water erosion (Wezel et al. 2000). They can hence offer diverse ecosystem services and goods to enhance the livelihoods of local populations (Breman and Kessler 1997). Moreover, better knowledge of tree dynamics constitutes a priority in order to better assess long-term changes in drylands, since they are “slow variables” which are considered as requiring more thorough surveys in such environments (Carpenter and Turner 2001; Blanco et al. 2015). Considering the crucial ecological role of trees in dryland ecosystems (Belsky et al. 1989), monitoring tree regeneration may allow more significant “slow” indications on potential degradation and desertification, or biological recovery.
In terms of development, the myth of the forest still persists, even in arid environments, as an indicator of health for the ecosystems. Although scarce and poor vegetation is a major structural component of arid zones, the idea of the necessity of stopping the advance of deserts by creating effective green barriers at their fringes is not new. Large-scale, and usually highly mediatized, restoration actions involving tree planting have produced mitigated results, such as the Great Green Wall of China (Wang et al. 2010; Cao et al. 2010), the Algerian green barrier in the 1970s (Bensaid 1995), and more recently the African Great Green Wall initiative (Dia and Duponnois 2010; Escadafal 2011). They classically include forest belts (10 to 20 km width) intended to: 1) stop sand movements, 2) improve ecological conditions, and 3) promote livelihoods. Despite the claim of participation and consultation with local populations, these projects are mainly designed from a technocratic perspective with little consideration for the perceptions, knowledge, and know-how local populations have developed in these constraining environments (Davis 2005; Wang et al. 2010).
Integrating both ecological engineering priorities and the culture and livelihoods of local societies in designing sound schemes for sustainable development in desertification contexts is not easy (Genin et al. 2006). Hence, learning more about small-scale local experiences would appear to be equally useful as the development of major projects. Moreover, for about twenty five years, the market value of the spontaneous products stemming from the local biodiversity has been perceived as an effective means to preserve not only the natural resources which are the basis of these products, but also the ecosystems and the ecological services they supply (Plotkin and Famolare 1992). The convergence between the preservation, patrimonialization and economic valorization of local biodiversity has allowed the progressive integration of local knowledge and know-how in the design of projects related to sustainable development. Local knowledge, as an integral part of the “natural” and “cultural” heritage, offers a basis for envisaging differently the modalities of biodiversity conservation and transmission (Folke 2004). The emblematic Moroccan argan tree (Argania spinosa (L.) Skeels) exemplifies this approach. This endemic tree found within an area of only 800 000 ha in south-western Morocco, is highly valued on the international market due to the interest of its oil for the cosmetic industry (Simenel et al. 2009). At the same time, it is a result of a process of co-evolution with local societies which developed refined practices for its management (McGregor et al. 2009; Genin and Simenel 2011). At the extreme south of its range of distribution, argan tree forest had almost disappeared, due to anthropogenic pressure and a very constraining climate (Alifriqui 2004). Land use changes occurring throughout contemporary history usually illustrate the dynamics of livelihoods in relation to the types of resources that are particularly sought after by local societies (charcoal production, cropping, hunting, livestock rearing), human movements and dependence upon these highly constraining arid areas (Lybbert et al. 2011). As commonly mentioned in the literature, vegetation change usually follows land use dynamics in the medium and long term (Tasser et al. 2007). Agricultural intensification or abandonment of agricultural fields are the main opposite drivers of agrarian changes (Tscharntke et al. 2005; Otto et al. 2006; Cramer et al. 2008). The pattern presented here is quite different, since both phenomena occur at the same time: the abandonment of unviable agricultural speculations associated with massive human migration, and the establishment of a system of cultivation requiring only occasional attention.
The relationships between agricultural practices and tree and forest development in arid or semi-arid zones are poorly documented. Some studies in the sub-Saharan zone of the Sahel have highlighted the interest of agroforestry parklands with regard to the social ecological resilience of the region (Petit 2003). Blanco et al. (2015) showed how occasional cereal cultivation promotes natural regeneration of Acacia raddiana Savi (Verchellia) in the Moroccan Saharan zone. Even less is known about biotic interactions between cultivated and spontaneous plants in these environments (Bountin and Jobin 1998). The prickly pear (Opuntia ficus-indica (L.) Miller), a cactus cultivated for fruits, fodder and oil production, embodies the paradox of being considered both as an invasive plant (Vila et al. 2003) and as a very useful plant to cultivate in arid zones (Le Houerou 1996; Novoa et al. 2015). This Mexican native plant is nowadays found in most of the arid and semi-arid areas of the world, where it plays a variety of roles within dryland farming systems (Shackleton et al. 2011). Prickly pear (this generic term includes different species (192) from the genera Opuntia) introduction has resulted in a dramatic encroachment in certain regions such as South Africa, Australia and Spain, where it is considered as invasive plant and combated as such (Shackleton et al. 2011). Various studies have demonstrated the negative effect on native plant biodiversity, mostly in semi-arid regions with precipitation between 300 and 600 mm year–1 (Zimmermann and Moran 1991). In North Africa, programmes for the extensive introduction of prickly pear cultivation (O. ficus-indica species) for providing forage and combating desertification, have received substantial funding from International Organizations. It is noteworthy that academic studies are highly sectorialized (ecological or agricultural studies), and poorly interconnected, which hinders a more global overview of the issues related to the prickly pear (Middleton 2002).
We report here a case not previously described of knock-on effects of speculative agricultural development in enhancing environmental conditions. The development of prickly pear cultivation and the dynamics of land uses and livelihoods during the past fifty years in south-western Morocco (Sidi Ifni Province) have generated extensive changes in both the ecological and socio-economic landscape. The aim of the present study was: 1) to assess the main social and ecological changes that have occurred during the past fifty years in the region of Sbouya-Mesti (Sidid Ifni Province), and 2) to identify the relationships between the age of prickly pear plantings and the regeneration of the argan tree, as well as some of its facilitation factors (degree of continentality, soil organic matter, mycorrhiza). We hypothesized that prickly pear cultivation, as perennial planting, due to its simplicity of implementation and coupled with the exclusion of livestock grazing, creates favorable conditions for biological recovery and the biological reactivation of argan stumps.
2 Materials and methods
2.1 Study site
This study took place in south-western Morocco, in Sidi Ifni Province (Fig. 1). It covered a surface area of about 350 km² between the localities of Arbâa Mesti-Sbouya and Sidi Ifni (29°38´N, 10°17´W). With mean annual rainfall of 150 mm and temperatures between 2 °C and 45 °C, the climate is Mediterranean arid, with mild winters, due to the proximity of the Atlantic Ocean (meteorological data from the Forest services of Sidi Ifni). However, annual variations in precipitation are very wide. During the winter 2014–2015, before the present study, rainfall were 204 mm in the Municipality of Mesti, which led to a spectacular development of annual plants, an uncommon situation in the region. Another point is the atmospheric humidity resulting from Atlantic fogs, which can supply significant, but as yet unquantified, amounts of water. The main geomorphological formation is represented by small mountain massifs forming part of the Anti-Atlas mountains, separated by hilly plains. The vegetation is of the infra-Mediterranean type, characterized by a thermophilous flora composed of scrubs and succulent plants (mainly Euphorbia officinarum subsp. echinus (Hook. f. & Coss.) Vindt, Euphorbia obtusifolia subsp. regis-jubae (Webb & Berthel.) Maire, Caralluma spp. etc.), shrubs (Launea arborescens Murb., Ziziphus lotus (L.) Lam., Periploca laevigata Aiton), and the presence of Argania spinosa (L.) Skeels (Msanda et al. 2002). The cultivated prickly pear (spineless variety) covers large areas in the region (Barthes et al. 2016). Scattered argan trees sometimes emerge from the landscape.
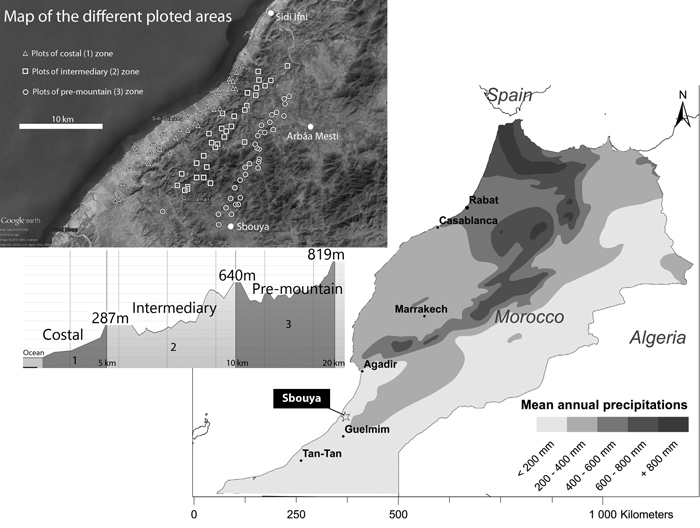
Fig. 1. Localization of the study area, climatic and geomorphologic context, and localization of plots sampled. View larger in new window/tab.
The region is populated by Berber-speaking sedentary peoples, from the Aït Ba’Amarane Tribe. They were colonized by the Spanish from 1934 to 1969. Their main sector of activity is traditionally rain-fed extensive agriculture, associated with small stock farming. However, the particularly harsh droughts of the 1970s led large numbers of people to emigrate to neighboring or more remote towns. Hence, the density of the permanent rural population drastically declined to fewer than 20 inhabitants per km² during the last thirty years (Barthes et al. 2016). Nowadays, human settlements are composed of small villages, mainly inhabited by the elderly and children (grandchildren), and where reduced-size flocks of goats and sheep still continue to be reared on abandoned fields and surrounding rangelands. The household economy is of family type, with diversified sources of income (agriculture, employment in towns), shared at family level. Locally, economic activity occurs mainly in summer when the active population comes back from towns to pick prickly pear fruits, and sell them to middlemen.
Prickly pear cultivation has undergone intensive development over the past forty years, covering nowadays more than 50% of the total area in some municipalities, such as Sbouya (Barthes et al. 2016).
2.2 Study species
2.2.1 Argan tree
The argan tree is a xerophilous and endemic species and is the only representative species of the tropical Sapotaceae family in Morocco. Its range of distribution covers about 800 000 ha under arid and semi-arid environment, with annual rainfall ranging from 100 to 300 mm. It is widely distributed from sea level up to 1500 m a.s.l. The argan tree is well adapted to semi-arid conditions, exhibiting physiological and morphological adjustments in response to drought (Chakhchar et al. 2015). Adult trees can survive in fairly dry climates. Further south, under the Saharan climate, when rainfall decreases to less than 100 mm, the argan tree can still grow following temporary water courses (M’Hirit et al. 1998).
It is a slow-growing tree, multi-branched from the base, that may be either shrub-like or a tree reaching 7–10 m in height. In Morocco, the argan tree provides essential ecological and economic functions for the local populations: protecting soil from desertification or from being used as grasslands, while valuable oil is obtained from the seeds (M’Hirit et al. 1998). It plays an important role in the local economy, mainly for its much-praised oil used nowadays in the cosmetics industry, for medicinal purposes and as food oil, but also as a major component for local livelihoods (Simenel et al. 2009). Argan tree forests, in their diversity, include more than 1000 plant species, of which 140 are endemic to Morocco and represent 29% of Moroccan flora, making the argan tree forest one of the most important ecosystems for Moroccan biodiversity (Benabid and Fennane 1994). However, this ecosystem is said to be in danger. Alifriqui (2004) reported that 240 000 ha of argan forest disappeared between 1918 and 1945 because of excessive anthropic action, leading to the deforestation of argan trees. Pressure on this environment has continued up to the present, due to intensive agricultural and urban expansion. Various authors have pointed out the urgent necessity of implementing effective protection plans in order to protect this unique ecosystem (Le Polain de Waroux and Lambin 2011).
2.2.2 Prickly pear
The prickly pear was implanted in Morocco around the end of the 16th century and comes from the Mexican drylands. It is a CAM (Crassulacean Acid Metabolism) species, particularly well-suited to the southern Morocco arid environment where soils are poor in nutrients, and because of its capacity to accumulate water in its spongy vegetative tissues. Prickly pear grows on light soils that do not retain water, making it a species particularly well-suited to the study area (Nerd et al. 1991).
Traditionally, products from prickly pear are fruits and cladodes for human and livestock feeding (Russell and Felker 1987). Today, in Morocco, prickly pear seed oil is also used in the cosmetic industry, and the fruits are sold in a dynamic national market, providing an additional income to local populations while allowing people to delineate plots and maintain land ownership. The prickly pear is the main plant cultivated in the study area, due to 1) its ease of implementation, 2) cultivation that does not necessitate the continuous presence of farmers, and 3) the production of diverse products (fruits, forage, oil), some with high added value.
Prickly pear (spineless variety) cultivation in Morocco occupies more than 150 000 ha, of which more than 40% is localized in the south-western Morocco, particularly the Sbouya-Mesti region under study. The size of prickly pear orchards is highly variable, from less than 1 ha to more than 100 ha, sometimes covering almost the whole of a hill. The main general data concerning this cultivation are as follows: mean production of 8 tons of fruits per hectare (0.5 to 1.2 € kg–1 sale price, depending on variety); 1 ton of fruit required to produce 1 kg of prickly pear seed oil, the selling price of which in the European market is around 800 € kg–1; 6500 tons of cladodes are sold each year for livestock feeding.
The life-span of productive orchards is between 35 and more than 50 years. We observed on several occasions the replacement of old orchards by grubbing up old plants and replanting new cladodes in vacant areas, and conserving argan tree resprouts.
Ecologically speaking, prickly pear species are the focus of controversy. In most parts of the world, they are viewed as invasive plants which encroach upon extensive areas. The issue of invasive plants has been the focus of particular attention among scientists since they can have a dramatic impact on native plant biodiversity, threaten the survival of rare and useful plants, and change drastically the overall biotic and abiotic conditions of local environments (Richardson et al. 2000; Levine et al. 2003). However, some studies point out the social and ecological interest of cultivated prickly pear, particularly in dry areas (Middleton 2002; Schackleton et al. 2011), and others highlight their potential role as nurse plants that enable other species to grow in desert areas (McAuliffe 1984; Mandujano et al. 2007; Neffar et al. 2011).
2.3 Methodology
2.3.1 Compartmentalization of the study area
On the basis of the geomorphology and distance from the ocean, the study area was compartmentalized in three distinct zones:
- a Coastal zone, characterized by a plain composed of abandoned fields and episodic cereal crops, according to the available annual rainfall (Coastal, zone 1)
- a hilly and plateau zone, where the oceanic influence is still present, with frequent fogs (Intermediary, zone 2)
- a pre-mountainous zone, at the bottom of a mountain range protecting the area from the Saharan influence (Pre-mountain, zone 3).
Distances from the Atlantic ocean are 0–5, 5–12, and 8–25 km, respectively. The zones are divided by two coastal mountain ranges, ranging from 0to 650 m a.s.l. In each zone, prickly pear orchards were classified in four age groups: 0–10 years, 11–25 years, 26–50 years and more than 50 years, on the basis of owners’ information and of two factors: i) presence of lignified cladodes (cactus around 20 years old), and, ii) number of cladodes’s floors on the cactus (one floor per year), as reported by Boujghagh and Chajia (2001). Depending on the age of the prickly pear plantation, the general physiognomic aspect can drastically change (Fig. 2). In zone 1, old orchards were difficult to find, due to more intensive historical agricultural pressure (mechanized plowing) and more recent abandonment of cereal cultivation.
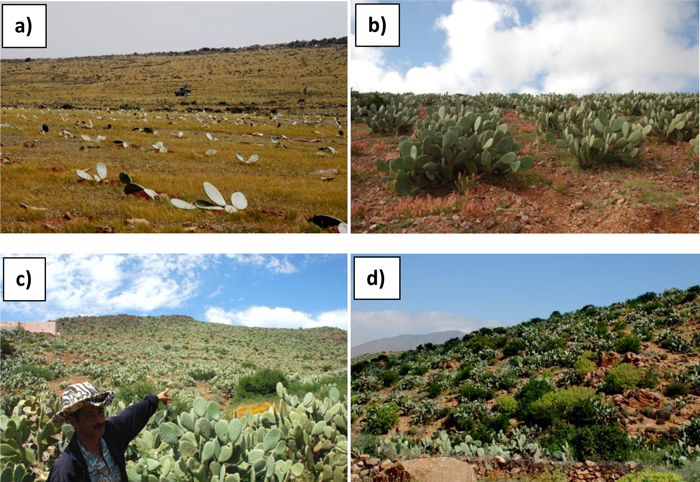
Fig. 2. Dynamics of prickly pear orchards: a) on-going prickly pear planting in an abandoned cereal field; b) 10-year-old prickly pear orchard; c) 25-year-old prickly pear orchard with argan tree regeneration (dark green patches); c) old prickly pear orchard (>50 years) with substantial argan tree regeneration.
2.3.2 Exploratory survey of agrarian dynamics
A first exploratory survey was performed to understand the main environmental and socio-economic dynamics that occurred in this area during the last century. Its first aim was to provide elements of lecture and understanding of the current features and landscapes of the region. Apart from the analysis of the rare available literature, semi-structured interviews were carried out with 28 local farmers (mainly male household Heads), by means of ‘snowball’ sampling (Biernacki and Waldorf 1981), since a more formal random sampling process based on household name lists was difficult to implement due to the high level of absenteism in the area. In addition to providing precise information on the age of plantation of previously distinguished parcels, interviews were conducted on the basis of a survey grid covering the following 4 themes: 1) agricultural practices associated with prickly pear cultivation, from installation to harvesting, 2) reasons for cultivating prickly pear, 3) dynamics of land use in the region, and 4) perceptions of environmental changes associated with prickly pear cultivation. Other stakeholders were also interviewed (local authorities, agricultural and forestry departments, cooperatives, factory managers), on the basis of the same survey grid. Data from available population and agricultural censuses (data base from Ministry of Agriculture) were also used to retrace the regional trends regarding rural occupation patterns in the region.
2.3.3 Regeneration of argan trees in prickly pear orchards
We selected ninety six plots with different ages of prickly pear plantation, from recently implanted prickly pears to prickly pear orchards of more than fifty years old, using a stratified sampling depending on the three zones and the four age classes (eight repetitions per situation). In each plot, ten 20 × 5 m vegetation bands were randomly installed, following the method proposed by Etienne (1989) for Mediterranean shrubland characterization. In each plot, we collected data concerning: 1) number of prickly pear individuals, and 2) number of argan tree regenerations (resprouts or seedlings), as well as 3) height of argan trees, 4) argan tree canopy diameter, and 5) nearest distance between two argan trees, for later analyses. This relatively high number of vegetation band repetitions per plot (10) may at first be viewed as excessive, but, due to: 1) the absence of any previous study on the topic, 2) the very arid conditions of the region, 3) a two-fold system of cacti planting (in line or randomly), and 4) apparently unclear distribution pattern of argan trees, we opted for this number of band repetitions in order to be sure of finding a sufficient number of argan trees for statistical analyses.
2.3.4 Organic matter in soil
Twenty-seven stations were selected in each of the three geographical zones and depending on the age of prickly pear orchard (three repetitions each): control (no Opuntia), “young” Opuntia field (10–25 years), and “old” Opuntia field (> 40 years). After confirmation of the exact age of these fields according to owner’s information, 500 g of soil was collected in each sample location at 0–20 cm depth in proximity to a prickly pear (50 cm to 1 m away). Total organic carbon content was measured according to carbon oxidation by potassium dichromate by means of Anne’s method described by Aubert (1978). One gram of fine soil (sieved at 2 mm) was mixed with 10 ml of potassium dichromate (K2Cr2O7); 5 ml of concentrated sulfuric acid was added, and left to cool for 30 minutes. Then the reaction was stopped by adding 100 ml of distilled water. After a settling period of 2 h, 25 ml of the supernatant solution was removed and 5 ml of orthophosphoric acid and 3 drops of diphenylamine (for coloring) were added. After stirring, the excess dichromate was titrated with a solution of iron sulfate and ammonium (Mohr’s salt concentration N / 2) until the solution turned green.
The percentage of total organic carbon (TOC) content in the dry matter was given by Eq. 1 (Aubert 1978):

where:
Vt = test titrate volume
Ve = sample titrate volume
F = correcting factor (3.9)
P = soil dry mass (g)
Data was then converted into a percentage of organic matter (%OM) by Eq. 2 (Dabin 1967):
![]()
2.3.5 Mycorrhizal fungi assessment
Soil samples were collected from the rhizosphere of O. ficus–indica; they were taken from 3 individual plants. Each sample consisted of about 1 kg of soil collected at 20 cm depth, at a distance varying between 10 to 50 cm from the base of the cactus, in order to avoid as far as possible contamination from the rhizosphere of other species. Control samplings were collected in prickly pear-free areas. All the soil samples were carefully mixed and the mycorrhizal fungi spores were extracted from the soil using the Gerdemann and Nicholson method (Gerdemann and Nicholson 1963). One hundred grams of dry soil was wet sieved on 500 to 50 µm mesh sieves and centrifuged in a water sucrose solution (60% weight/volume) for 15 min at 3000 rpm. Then the supernatant was poured through a 50 µm sieve and rinsed with tap water. Spores were counted under a stereomicroscope and grouped according to their morphological characteristics. Spore size and colour were assessed in water under a stereomicroscope (Olympus SZ H10 research stereomicroscope).
2.3.6 Data analysis
Data from interviews were qualitatively processed, following an ethno-ecological approach (Ruiz-Mallen et al. 2012), to highlight knowledge on perception and practices concerning prickly pear cultivation, and its dynamics. They constituted the knowledge base for reconstructing past land use dynamics, and projecting possible landscape trajectories. To this end, we redrew historical physiognomic diagrams of the vegetation and landscape, following descriptive schemas proposed by Whittaker (1975), and inspired by structure models proposed by Couteron et al. (1996) for ligneous vegetation.
Concerning measurements, means and standard deviations of the measured variables were calculated for locations and ages of prickly pear orchards. One and/or two-way ANOVAs were applied to test the effect of localization and age of orchards on argan regeneration, soil organic matter content and mycorrhiza spores. Data concerning the number of argan tree regenerations and percent of organic matter were transformed by Logarithmic and Arc sinus models, respectively, in order to fit with normality requirements. Student-Newman-Keuls test calculated at p < 0.01 was applied to test differences between variables for each orchard age. Bivariate Pearson correlations and regression analyses were done between age of orchard and number of argan tree regenerations with linear and logarithmic models. All statistical analyses were carried out using SPSS software 12.0 for Windows (SPSS Inc., Chicago, USA).
3 Results
3.1 Agrarian system dynamics and comparative advantages of prickly pear cultivation
3.1.1 Land use changes
The narratives of the interviewees concerning the agrarian changes presented a remarkable homogeneity. They provided the opportunity for qualitatively retracing the main features that have occurred during the fifty past years, as well as the reasons behind these dynamics. Four main phases occurred:
The first phase (first half of the 20th century) concerns a massive deforestation of the argan forest which covered most of the territory. In those days, the argan tree was not valued for oil production, which was considered as local food (“beldi”). Nevertheless, charcoal was in high demand, and, in particular argan charcoal, due to its high caloric power.
The second phase (from the 2nd World War until 1969), included the Spanish Protectorate period, when the Spanish forestry authority impeded tree cutting with the aim of slowing down the spread of the desert. However, illegal cutting and military cutting still continued, with the development of cereal crop cultivation and goat herding. The prickly pear was present, but mainly confined to the ‘Ourtis’ (family gardens for green vegetables and fruits), localized close to houses. Prickly pear was used as a food reserve for humans and animals for dry years.
At independence (1969 in Sidi Ifni), laws regulating forest uses were almost unchanged, but controls were almost non-existent, and extensive areas were sown for hypothetical barley production, even on publicly owned lands (phase 3). This model rapidly turned out to be unviable, and massive emigration started in the middle of the 1970s. For example, in the rural Commune of Sbouya, censuses indicated a reduction of the permanent population from 24 237 in 1974 to 5028 in 2004. Between 1970 and 1994, field occupation for barley cultivation decreased from 80 to 35% of the total surface area (data base for Ministry of Agriculture), and this trend has continued and intensified up to the present. Nowadays, only small fields are sown with cereals near farms, with episodic cultivation during particularly wet years. At the same time, areas planted with cacti increased dramatically: from 1987 to 2011, for example, areas planted in cactus rose from 2390 to 3720 ha (+56%) in the municipality of Sbouya and from 490 to 2810 ha (+473%) in the municipality of Mesti. Earlier data are difficult to collect due to the rarity of censuses and the extensive changes in administrative boundaries.
More recently, development plans have emerged with the aim of encouraging the cultivation and the marketing of prickly pear products (phase 4). This has resulted in the development of an infrastructure of roads and passable tracks, cooperatives, and private business initiatives with irrigated orchards for intensive production. However, the main feature is extended orchards owned by non-residents who come back in summer to harvest fruits and sell directly to middlemen. Almost no agricultural work is done on the orchards except for the replacement of old prickly pear specimens.
According to the farmers interviewed, the argan tree is commonly perceived as re-emerging in prickly pear orchards (20 on 28 people observed this phenomenon). They mentioned that prickly pear serves as protection for the argan tree, both by the exclusion of livestock which intensively browse argan trees, and by creating micro-climatic conditions favorable for its regrowth. Old men mentioned that this just represents a return to the landscapes they used to see when they were children, with an additional component: the prickly pear. They unanimously consider this regeneration as beneficial for them and a sign of “good health” of the soil. Some of them have started to shape argan tree resprouts by cutting unnecessary resprout twigs and encouraging tree port configuration, with the aim of future nut production (10% of the panel).
Based on these narratives, Fig. 3 shows schematically the physiognomic changes which occurred during the 20th century, and on the basis of the rare emerging cases we observed, aims to display what could be in future a novel form of stable agro-forestry parklands, composed of both productive argan trees and prickly pears.
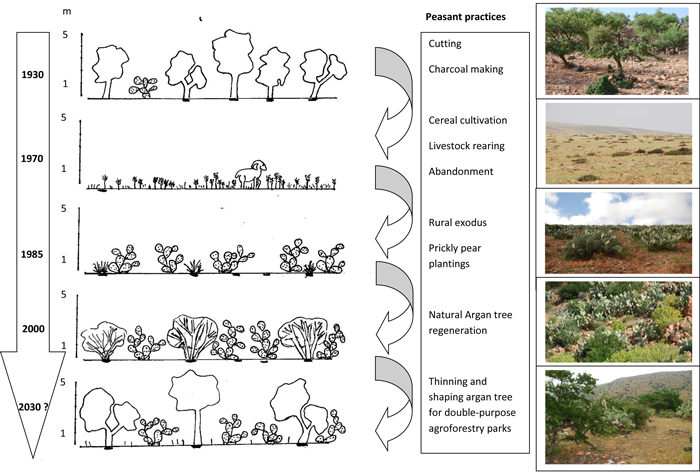
Fig. 3. Vegetation physiognomic diagrams associated with land use changes during the 20th century in the hinterland of Sidi Ifni (Morocco).
3.1.2 Comparative advantages of prickly pear cultivation
Prickly pear is unanimously perceived by farmers a sort of ‘miraculous’ plant, well adapted to climatic conditions of the region and very useful for livelihood improvement (100% of the panel). They all pointed out its characteristic of being very easy to implement. Farmers place previously slightly sun-dried plants composed of three to four cladodes directly on the ground, and immobilize them with a heavy stone. There is no necessity for soil removal, nor use of fertilizer. The spineless variety is strongly preferred, due to facility in harvesting fruits. However, orchards are usually encircled by hedges composed of thorny varieties of prickly pear (locally called Achefri) to prevent access by small ruminant flocks. Agricultural authorities promote a more mechanized mode of plant installation by digging furrows in fields, and planting in line (Agricultural Service of Sidi Ifni, pers. comm.).
There is no cultivation operation involved for the planting process. Harvesting occurs mainly in summer (Aïssa variety), and sometimes in autumn for the Moussa variety (big juicy fruits), which is specific to the region, and highly valorized in the national market. This ease in managing the cultivation has promoted its success with the local population, who mostly moved to towns in the 1980s.
Only three of them stressed on the huge development of this culture ‘until the top of the hills’ and interrogated themselves: ‘what if a disease occurs with such extensive areas?’. None of them perceived any risk of the extension of this culture for the local biodiversity, more they argued that there is higher plant diversity in prickly pear orchards than in surrounding relictual rangelands.
3.2 Relationships between age of prickly pear orchards and argan tree regeneration
Regeneration of the argan tree is mainly in the form of coppicing, from ancient stumps. However, we observed regeneration by seedling in a proportion of between 0and 4% among the sampled plots.
The total number of argan tree regenerations varied from 0to 42 per 1000 m², depending on the age of the orchard. Argan regeneration was almost non-existent in zone 1, while no differences were found for zones 2 and 3 (F = 1.2; p > 0.05) (Fig. 4A). Statistical tests of homogeneous age groups showed a clear positive relationship between the age of the prickly pear orchard and the number of argan regenerations (F = 174.0; p < 0.001), but there would seem to be a tray starting from the 25–50 -year-old prickly pear group (Fig. 4B). We hence pooled data from zone 2 and zone 3 for correlation analyses. Correlation curves showed a significant close correlation between the number of argan tree regenerations and the age of prickly pear plantation (Fig. 5). The determination coefficient (R²) varied from 0.65 (logarithmic model, p < 0.001) to 0.76 (linear model, p < 0.001), indicating a highly significant positive effect of prickly pear plantation on the reappearance of argan trees.
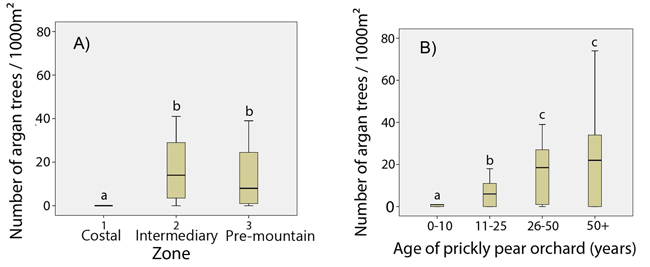
Fig. 4. Number of argan tree regeneration A) by zone (degree of continentality); B) by age class. Significant differences are marked with different letters at p < 0.01 (Student-Newman-Keuls test).
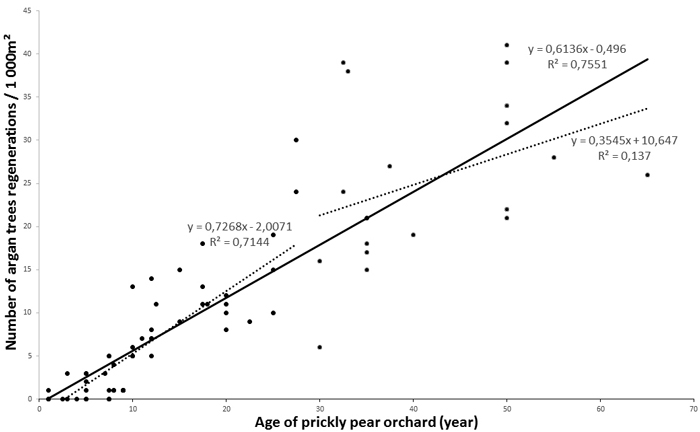
Fig. 5. Regression curves between age of prickly pear orchard and argan tree regeneration. View larger in new window/tab.
3.3 Organic matter
The crude percentages of organic matter in soil varied from 0.19% (in a control plot) to 3.27% (in a > 50-year-old plot), but with high variations among plots. However, results showed that there was a significant difference between the three zones (continental or coastal), with more organic matter in the two continental zones than in the coastal zone (F = 6.13; P = 0.003). Age of plantation also positively affected soil organic matter content in all these three zones (F = 16.9; P < 0.001). Statistical tests showed that there are three groups according to organic matter: the first (with levels of organic matter of 0.71% on average) in control fields without prickly pear, the second (intermediate levels of organic matter of 0.94% on average) in “young” fields around 10–25 years-old, and the last (with the highest levels of organic matter of 1.35% on average) in “old” fields more than 50-years-old (Table 1).
| Table 1. Effect of distance to the Atlantic Ocean and the age of prickly pear orchard on the Organic Matter content of soil (%). Values in brackets correspond to standard error (SE). Means with different superscript letters are statistically different at p < 0.01 in ANOVA (Student-Newman-Keuls test). | |||||
| Zone | |||||
| 1 Coastal | 2 Intermediary | 3 Pre-mountain | Mean | ||
| Age of prickly pear orchard (years) | Control | 0.51 (0.07) | 0.79 (0.14) | 0.83 (0.27) | 0.71a (0.10) |
| 10–25 | 0.70 (0.23) | 1.13 (0.11) | 1.00 (0.11) | 0.95b (0.09) | |
| >40 | 1.26 (0.27) | 1.15 (0.19) | 1.64 (0.26) | 1.35c (0.14) | |
| Mean (SE) | 0.82a (0.13) | 1.03b (0.09) | 1.16b (0.14) | ||
3.4 Total spores number per 100 g soil
The total number of mycorrhizal spores per 100 g of soil isolated from the rhizospheric soil of O. ficus-indica varied between 284 and 2044 in the control soil away from the influence of O. ficus-indica, 215 and 858 in the soil of 10–25 year-old plants, 355 and 1191 spores in the rhizospheric soil of 40-year-old plants. The results from the three zones (1 coastal and 2 continental) did not show any significant differences between the different zones (Table 2). The only significant difference was noted between soils under Opuntia and those away from its influence. The abundance of the annual flora at the time of the sampling led to an increase in mycorrhizal propagules in the soil, due to the non-specificity of the endomycorrhizal symbiosis involving the annual plants as well as the perennial O. ficus-indica. Similarly, the age of the plants did not show a significant influence on the mycorrhizal presence in the soil, measured as the total number of spores in 100 g of rhizopheric soil.
| Table 2. Effects of age of prickly pear orchard on the total number of arbuscular mycorrhizal fungi spores in rhizosphere soil of O. ficus-indica (number of spores/100 g soil, and standard error (SE)). Means with different superscript letters are statistically different at p < 0.05 in ANOVA (Student-Newman-Keuls test). | |||||
| Zone | |||||
| 1 Coastal | 2 Intermediary | 3 Pre-mountain | Mean | ||
| Age of prickly pear orchard (years) | Bare soil | 878 (206) | 935 (163) | 726 (45) | 835a (87) |
| 10–25 | 534 (66) | 543 (33) | 497 (93) | 522b (42) | |
| >40 | 592 (85) | 633 (90) | 453 (51) | 550b (44) | |
| Mean (SE) | 668a (80) | 704a (72) | 559a (44) | ||
4 Discussion
Local landscape appeared to have suffered drastic changes during the contemporaneous history; particularly trees used to have played a stronger role in past farming systems in this arid region. Narratives of interviewed farmers are in complete accordance with the description given by Hart (1973), after his field study in 1962, which highlighted the type of landscape found in those days a follow:
“…the land of the Ait Ba ‘Amran is rolling, hilly and studded with argan groves, while heavy thickets of scrub in the Imstiten (Misti) area provide ideal cover for herds of wild boar which are said to abound there to such an extent as to be a menace to the crops. As elsewhere in Morocco, the staple crop is barley, with some wheat. But the main supplements to barley are twofold: the nuts of the argan tree, from the pips of which is extracted that other now expensive staple in the Susi [Region of Souss] diet, argan oil; and the fruit of the prickly pear cactus, a plant of Mexican origin, which was introduced by the Spaniards to Northwest Africa in the 16th century, by way of the Canary Islands, whence its local name is aknari. Of the domestic animals, goats predominate, and then sheep, cattle, donkeys and camels, in that order. All use the “suspended pasturage” afforded by the argan trees, although here the goats have cornered the ecological market” (p. 63).
These drastic land use changes undoubtedly modified the development of spontaneous vegetation and affected biodiversity, but reserved some surprises concerning the renewal of the native argan tree stands.
The relationship between the age of the prickly pear plantation and argan tree regeneration was formally highlighted here for the first time, although it had been previously suggested but not quantified by Aafi (2007). It is worth noting the extreme resistance of the argan tree, which is capable of sprouting from the stump, sometimes more than thirty years after having been cut down. We find here one the characteristics of plants from arid zones which have developed very elaborated mechanisms of dormancy in order to be capable of renewing their vegetative activity when the conditions of the environment are more favorable (McDowell et al. 2008). Similar mechanisms associated with sprouting were described by Del Tredici (2001) for temperate trees. However, the regeneration we observed here seems to be only a knock-on effect of a certain preservation of the soil because of the implantation of the prickly pear which does not require removal of the soil, and the imposition of a drastic reduction of pastoral pressure to protect the orchards. The regeneration of the argan tree was not ex ante the intention of the farmers. Hence, absence of grazing, and improvement of soil fertility – perhaps associated with facilitation factors linked with interactions between plants – resulting from the proximity of prickly pear, appeared to be the major drivers for argan tree renewal. The low regeneration of argan trees in zone 1 is difficult to interpret in terms of edapho-climatic conditions of the environment because the argan tree is considered as a highly plastic species within its range of distribution (Neffar et al. 2011). Moreover, in northern parts of its range, the argan tree grows equally well near the Atlantic coast or inland. Again, the intensity and temporality of past land use for cereal cultivation in these flatter areas, and hence mechanical plowing that is highly destructive of tree stumps, would no doubt be the main explanation for this feature.
In immediate neighboring environments, such as the municipality of Lakhass, where prickly pear development has not been as extensive as in the Sidi Ifni Region, it seems that this process has not occurred (Alahyane 2004). The argan tree is nowadays the focus of a great deal of interest, both in economic and ecological terms (Lybbert et al. 2011). Its regeneration is problematic, and research has so far been unable to find technical solutions enabling efficient large-scale sowing (Bellefontaine et al. 2010). The case we have described here could be one basis for conservation plans for this emblematic and endangered species, while providing other ecosystem services involved in combating desertification. Agroforestry parklands locally implanted directly by the local population could also provide a non-negligible source of income, due to the high economic value of products derived from both the argan tree and the prickly pear.
Results concerning the soil organic matter content showed the positive impact of the prickly pear on surface layers of soils, increasing as much as two-fold the OM content in old prickly pear orchards. This finding illustrates the role prickly pear can play in stimulating Argan tree regeneration. Other authors have also shown a significant increase of organic matter in Opuntia fields compared to empty fields in this kind of environment (Vasquez-Méndez et al. 2009; Neffar et al. 2011). This enrichment in organic matter, apart from increasing plants’ mineral nutrition (Austin et al. 2004), leads to the emergence of larger and more stable aggregates in soils, which reduce the volume density of the soil, hence becoming more airy, and facilitating soil biological activity (Vasquez-Mendez et al. 2009). These ‘re-fertilized’ islands allow easier installation of other plant species, which find more favorable conditions, promoting local biodiversity (Alifriqui, unpublished data). Even if other factors may have intervened in interaction, Opuntia spp. seems to be a suitable ‘nurse’ plant in these drylands because of its effect on the composition and structure of degraded soils, as reported for other Mediterranean dryland plants (Soliveres et al. 2012). It fully participates to the multiple and complex interactions observed for facilitation in arid environments (Michalet 2010), by creating favorable conditions for argan tree development (Fig. 6).
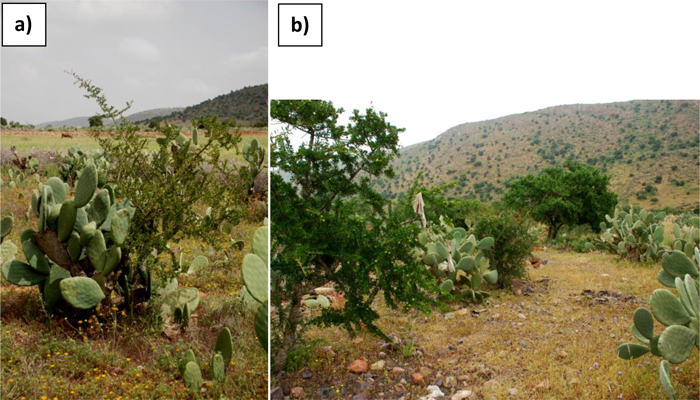
Fig. 6. Interactions between prickly pear orchards and argan tree regeneration: a) prickly pear as a plant nurse?; b) the future of regional landscape?: double-purpose agroforestry parkland composed of prickly pears and thinned argan trees for nut production.
The exceptional abundance of annual plants at the date of sampling contributed to enriching the soil with fungal propagules, while the density of the roots and their localization in the upper layer of the soil allowed a greater development of mycorrhizal fungi. Hence, spore density was revealed to be higher outside the area of influence of prickly pear than within it. These fungi are, however, more exposed to environmental constraints and their activity is highly dependent on rainfall. Hence, results from this preliminary study will have to be balanced with others based on climatic conditions more commonly found in the region. However, the numbers of endomycorrhizal fungal spores measured in the rhizospheric soil of prickly pear are comparable to those obtained in other studies under Mediterranean conditions. Studies conducted in arid and semi-arid Mediterranean regions on many plant species representative of those ecosystems (Stipa tenacissima L., Pistacia lentiscus L., Rhamnus lycioides L., Olea europaea L., Retama sphaerocarpa (L.) Boiss., Lavandula spica L., Thymus spp.) have shown that those species first affect their own habitat by creating microenvironments characterized by a higher organic matter content and an improvement of the soil structure (Caravaca et al. 2003; Ouahmane 2007). These plants are thus better protected through higher resistance to different forms of stress, such as drought and nutrient deficiency in its environment.
The study of the mycorrhization status of these species has shown that the number of mycorrhizal fungi spores and the length of mycorrhized root fragments and mycelia are significantly higher than those without vegetation cover. These plants are said to be nurturing species and are characterized by high levels of mycotrophy; they act as propagation vectors for fungal propagules (Ouahmane 2007; Requena et al. 1996). The fertility islands formed by these plants have a high level of biological activity and the extrametrical mycelium is the functional component for the development and functioning of these islands (Moro et al. 1997). The loss or decrease of fungal propagules from symbiotic mycorrhizae can limit both natural and artificial regeneration (Requena et al. 2001).
The analysis of the mycorrhizal infection potential has shown that many types of fungal propagules such as spores, mycorrhized roots or fungal mycelia present in the soil, can generate a mycorrhiza. Spores are more resistant forms that can remain in the soil for months or even years until the environmental conditions and the presence of plant roots allow for their germination. These different types of propagules have a strong biological potential by allowing the propagation of a plant’s mycorrhization from one plant to another or from one soil to another. Further studies will have to shed more light on the potential symbiotic relationships between the two species under study, as well as the types of mechanisms involved.
Finally, the question of the risks engendered by the massive introduction of the prickly pear with regard to local biodiversity will have to be detailed in greater depth for this arid area. A recent Master thesis study we directed on the same area, tended to indicate that global biodiversity was improved with the age of prickly pear plantation (from 41 species in control plots to 47 in old prickly pear orchards, in zone 1, from 55 to 73, respectively, in zone 2, and from 68 to 74, respectively, in zone 3) (Yous 2016). Our preliminary analyses did not reveal a deleterious effect of plant diversity in prickly pear orchards in this area of south-western Morocco in areas that had previously been disturbed. However, more in-depth botanical surveys, particularly on rare plants and plant functional types, are essential to provide further insight.
5 Conclusion
This study highlighted the significant positive relationship between the age of implanted prickly pear orchards and natural argan tree regeneration in the pre-Saharan zone of south-western Morocco. This relationship is mainly related to interconnected changes in traditional land uses and the activation of facilitation factors such as the improvement of the soil organic matter and nurse plant phenomena.
It has highlighted that some cultivation enterprises can, under certain circumstances, play a positive role in the improvement of ecological conditions and the promotion of local biodiversity. Moreover, combating desertification can also take inspiration from ‘success stories’ that may not involve major international technical projects. In order to conserve dryland biodiversity, support must be given to adapting local knowledge systems to the changing political, economic and environmental (including climatic) conditions (Gudka et al. 2014). Finally, between landscape domestication and enabling natural ecological processes to proceed, this case study has highlighted synergies that can be envisaged as a basis for promoting both sustainable development and ecological restoration, and can inspire alternative guidelines for more integrated plans for combating desertification.
Acknowledgements
This study was supported by the Project MED-INN-LOCAL (Local Innovations in Mediterranean Landscapes) of the French National Research Agency (Grant: ANR-12-TMED-0001), and by the International Mixed Laboratory MEDITER of IRD. Thanks are also due to two anonymous Reviewers for their suggestions, and to Michael Paul for revising English wording.
References
Aafi A. (2007). Role of cactus in the restoration of the argan forest and its flora and fauna components in the Province of Tiznit. Annales de la Recherche Forestière au Maroc 38: 69–76. [In French].
Adhikari B., Di Falco S., Lovett J.C. (2004). Household characteristics and forest dependency: evidence from common property forest management in Nepal. Ecological Economics 48: 245– 257. http://dx.doi.org/10.1016/j.ecolecon.2003.08.008.
Alahyane M. (2004). Anthropological studies in the Anti Atlas: the Lakhssas Tribe. Institut Royal de la Culture Amazighe, Rabat, Série: Etudes n°4. 136 p. [In French].
Alifriqui M. (2004). The ecosystem of the argan tree. United Nations Development Programme. PNUD, Rabat, Paris. 124 p. [In French].
Aubert G. (1978). Method of soil analysis. Centre national de documentation pédagogique, Centre régional de documentation pédagogique de Marseille. 632 p. [In French].
Austin A.T., Yahdjian L., Stark J.M., Belnap J., Porporato A., Norton U., Ravetta D.A., Schaeffer S.M. (2004). Water pulses and biogeochemical cycles in arid and semiarid Ecosystems. Oecologia 141: 221–235. http://dx.doi.org/10.1007/s00442-004-1519-1.
Barthes A., Baudot P., Alifriqui M., Michon G., Genin D., Kamil H., Romagny B., Simenel R. (2016). Innovative dynamics in the arid moroccan hinterlands: the case of prickly pear, an emerging territorial resource. In: Berriane M., Michon G. (eds.). Mediterranean terroirs: environment, patrimony and development in Morocco. IRD Editions & AUF Marseille. p. 145–158. [In French].
Bellefontaine R., Ferradous A., Alifriqui A., Monteuuis O. (2010). Vegetative propagation of argan tree, Argania spinosa in Morocco: the John Goelet project. Bois et Forêts des Tropiques 304: 47–59.
Belsky A.J., Amundson R.G., Duxbury J.M., Riha S.J., Ali A.R., Mwonga S.M. (1989). The effects of trees on their physical, chemical and biological environments in a semi-arid savanna in Kenya. Journal of applied ecology 26: 1005–1024. http://dx.doi.org/10.2307/2403708.
Benabid A., Fennane M. (1994). Knowledge on Morocco’s vegetation phytogeography, phytosociology and series of vegetation. Lazaroa 14: 21–97. [In French].
Bensaid S. (1995). A Critical review of the Green Dam in Algeria. Sécheresse 6(3): 247–255. [In French].
Biernacki P., Waldorf D. (1981). Snowball sampling: problems and techniques of chain referral sampling. Sociological Methods & Research 10(2): 141–163. http://dx.doi.org/10.1177/004912418101000205.
Blanco J., Genin D., Carrière S.M. (2015). The influence of Saharan agro-pastoralism on the structure and dynamics of acacia stands. Agriculture, Ecosystems & Environment 213: 21–31. http://dx.doi.org/10.1016/j.agee.2015.07.013.
Boujghagh M., Chajia L. (2001). Cactus: a drought management tool in southern Morocco. Terre et vie 52: 1–7. [In French].
Bountin C., Jobin B. (1998). Intensity of agricultural practices and effects on adjacent habitats. Ecological Applications 8(2): 544–557. http://dx.doi.org/10.1890/1051-0761(1998)008[0544:IOAPAE]2.0.CO;2.
Breman H., Kessler J.J. (1997). The potential benefits of agroforestry in the Sahel and other semi-arid regions. European Journal of Agronomy 7(1): 25–33. http://dx.doi.org/10.1016/S1161-0301(97)00035-X.
Cao S., Tian T., Chen L., Dong X., Yu X., Wang G. (2010). Damage caused to the environment by reforestation policies in arid and semi-arid areas of China. Ambio 39(4): 279–283. http://dx.doi.org/10.1007/s13280-010-0038-z.
Caravaca F., Alguacil M.M., Figueroa D., Barea J.M., Roldan A. (2003). Re-establishment of Retama sphaerocarpa as a target species for reclamation of soil physical and biological properties in a semi-arid Mediterranean area. Forest Ecology and Management. 182: 49–58. http://dx.doi.org/10.1016/S0378-1127(03)00067-7.
Carpenter S.R., Turner M.G. (2000). Hares and tortoises: interactions of fast and slow variables in ecosystems. Ecosystems 3(6): 495–497. http://dx.doi.org/10.1007/s100210000043.
Chakhchar A., Wahbi S., Lamaoui M., Ferradous A., El Mousadik A., Ibnsouda-Koraichi S., Filali-Maltouf A., El Modafar C. (2015). Physiological and biochemical traits of drought tolerance in Argania spinosa. Journal of Plant Interactions 10(1): 252–261. http://dx.doi.org/10.1080/17429145.2015.1068386.
Cramer V.A., Hobbs R.J., Standish R.J. (2008). What’s new with old fields? Land abandonment and ecosystem assembly. Trends in Ecology and Evolution 23(2): 104–112. http://dx.doi.org/10.1016/j.tree.2007.10.005.
Couteron P., Mahamane A., Ouedraogo P. (1996). Analysis of the structure of wood stands in a «stuffed tabby» north Yatenga (Burkina Faso). Current status and evolutionary consequences. Annales des sciences forestières 53(4): 867–884. http://dx.doi.org/10.1051/forest:19960406.
Dabin B. (1967). Application of automatic assays soil analysis: part 3. In: Cahiers ORSTOM (ed.), Série Pédologie 5(3): 257–263. [In French].
Davis D.K. (2005). Indigenous knowledge and the desertification debate: problematising expert knowledge in North Africa. Geoforum 36(4): 509–524. http://dx.doi.org/10.1016/j.geoforum.2004.08.003.
Del Tredici P. (2001). Sprouting in temperate trees: a morphological and ecological review. The Botanical Review 67(2): 121–140. http://dx.doi.org/10.1007/BF02858075.
Dia A., Duponnois R. (2010). The African major project of the great green wall: concept and application. Marseille, IRD Edition. 465 p. [In French].
Escadafal R. (Coord.) 2011. The African great green wall project: what advice can scientists provide? CSFD Topical issue. http://www.csf-desertification.org/great-green-wall.
Etienne M. (1989). Non destructive methods for evaluating shrub biomass: a review. Acta oecologica. Oecologia applicata 10(2): 115–128.
Folke C. (2004). Traditional knowledge in social-ecological systems. Ecology and Society 9(3): 7. http://www.ecologyandsociety.org/vol9/iss3/art7/.
Genin D., Simenel R. (2011). Endogenous Berber forest management and the functional shaping of rural forests in Southern Morocco: implications for shared forest management options. Human Ecology 39(3): 257–269. http://dx.doi.org/10.1007/s10745-011-9390-2.
Genin D., Guillaume H., Ouessar M., Ouled Belgacem A., Romagny B., Sghaier M., Taâmallah H. (2006). Between desertification and development: the Tunisian Jeffara. Cérés (ed.), IRD Tunis. 350 p. [In French].
Gerdemann J.W., Nicolson T.H. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological society 46(2): 235–244. http://dx.doi.org/10.1016/S0007-1536(63)80079-0.
Gudka M., Davies J., Poulsen L., Schulte-Herbrüggen B., MacKinnon K., Crawhall N., Henwood W.D., Dudley N., Smith J. (2014). Conserving dryland biodiversity: a future vision of sustainable dryland development. Biodiversity 15(2–3): 143–147. http://dx.doi.org/10.1080/14888386.2014.930716.
Hart D.M. (1973). The Ait Ba’Amran of Ifni: an ethnographic survey. Revue de l’Occident musulman et de la Méditerranée 15(1): 61–74. http://dx.doi.org/10.3406/remmm.1973.1227.
Le Houérou H.N. (1996). The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. Journal of Arid Environments 33(2): 135–159. http://dx.doi.org/10.1006/jare.1996.0053.
Le Polain de Waroux Y., Lambin E.F. (2011). Monitoring degradation in arid and semi-arid forests and woodlands: the case of the argan woodlands (Morocco). Applied Geography 32: 777–786. http://dx.doi.org/10.1016/j.apgeog.2011.08.005.
Levine J.M., Vila M., Ane Antonio C., Dukes J.S, Grigulis K., Lavorel S. (2003). Mechanisms underlying the impacts of exotic plant invasions. Proceedings of the Troyal Society B 270(1517). http://dx.doi.org/10.1098/rspb.2003.2327.
Lybbert T.J., Aboudrare A., Chaloud D., Magnan N., Nash N. (2011). Booming markets for Moroccan argan oil appear to benefit some rural households while threatening the endemic argan forest. Proceedings of the National Academy of Sciences 108: 13963–13968. http://dx.doi.org/10.1073/pnas.1106382108.
M’Hirit O., Benzyane M., Benchekroun F., El Yousfi S.M., Bendaanoun M. (1998). Argan: a fruit-tree species with multiple uses. Mardaga (ed.), Sprimont. 150 p. [In French].
Mace R. (1991). Overgrazing overstated. Nature 349: 280–281. http://dx.doi.org/10.1038/349280d0.
McAuliffe J.R. (1984). Sahuaro-nurse tree association in the Sonoran desert: competitive effects of sahauaros. Oecologia 64: 319–321.
McDowell N., Pockman W.T., Allen C.D., Breshears D.D., Cobb N., Kolb T., Plaut J., Sperry J., West A., Williams D.G., Yepez A. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178: 719–739. http://dx.doi.org/10.1111/j.1469-8137.2008.02436.x.
McGregor H.V., Dupont L., Stuut J.B.W., Kuhlmann H. (2009). Vegetation change, goats, and religion: a 2000-year history of land use in southern Morocco. Quaternary Science Reviews 28(15): 1434–1448. http://dx.doi.org/10.1016/j.quascirev.2009.02.012.
Mandujano M.C., Golubov J., Huenneke L. (2007). Effect of reproductive modes and environmental heterogenity in the population dynamics of a geographically widespread clonal desert cactus. Population Ecology 49: 141–153. http://dx.doi.org/10.1007/s10144-006-0032-2.
Michalet R. (2010). Is facilitation in arid environments the result of direct or complexe interactions? New Phytologist 169(1): 3–6. http://dx.doi.org/10.1111:j.1468-8137.2006.01617.x.
Middleton K. (2002). Opportunities and risks: a cactus pear in Madagascar. In: Nefzaoui A., Inglese P. (eds.). Proceedings of the 4th International Congress on cactus pear and Cochineal. Acta Horticulturae 581. p. 63–73.
Moro M.J., Pugnaire F.I., Haase P., Puigdefábregas J. (1997). Effect of the canopy of Retama sphaerocarpa on its understorey in a semiarid environment. Functional Ecology 11(4): 425–431. http://dx.doi.org/10.1046/j.1365-2435.1997.00106.x.
Msanda F., El Aboudi A., Peltier J.P. (2002). Particularities of the flora and vegetation in the South-Western Anti-Atlas (Morocco). Feddes Repertorium 113: 603–615. http://dx.doi.org/10.1002/fedr.200290008.
Neffar S., Beddiar A., Redjel N., Boulkheloua J. (2011). Effects of age of prickly pear plantations (Opuntia ficus-indica f. inermis) on the properties of soil and vegetation in Tebessa (semi-arid area in eastern Algeria). Ecologia mediterranea 37(1): 5–15. [In French].
Nerd A., Karadi A., Mizrahi Y. (1991). Salt tolerance of prickly pear cactus (Opuntia ficus-indica). Plant and Soil 137(2): 201–207. http://dx.doi.org/10.1007/BF00011198.
Novoa A., Le Roux J.J., Robertson M.P., Wilson J.R.U., Richardson D.M. (2015). Introduced and invasive cactus species: a global review. AoB PLANTS 7: plu078. http://dx.doi.org/10.1093/aobpla/plu078.
Ouahmane L. (2007). Roles of associated plants (Lavandula and Thymus) in the regeneration of the Atlas cypress: effects on rhizospheric diversity. Thèse de Doctorat National, Université cadi Ayyad, Marrakech. 234 p. [In French].
Otto R., Krüsi B.O., Burga C.A., Fernandez-Palacios J.A. (2006). Old-field succession along a precipitation gradient in the semi-arid coastal region of Tenerife. Journal of Arid Environments 65: 156–178. http://dx.doi.org/10.1016/j.jaridenv.2005.07.005.
Petit S. (2003). Parklands with fodder trees: a Fulße response to environmental and social changes. Applied Geography 23(2): 205–225. http://dx.doi.org/10.1016/j.apgeog.2003.08.008.
Plotkin M., Famolare L. (1992). Sustainable harvest and marketing of rain forest products. Island Press (ed.), Washington D.C. 325 p.
Requena N., Jeffries P., Barea J.M. (1996). Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Applied and Environmental Microbiology 62(3): 842–847. http://dx.doi.org/10.1128/AEM.67.2.495-498.2001.
Requena N., Perez-Solis E., Azcón-Aguilar C., Jeffries P., Barea J.M. (2001). Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Applied and environmental microbiology 67(2): 495–498. http://dx.doi.org/10.1128/AEM.67.2.495-498.2001.
Richardson D.M., Pysek P., Rejmanek M., Barbour M.G., Dane Panetta F., West C.J. (2000). Naturalization and invasion of alien plants: concepts and definitions. Diversity and Conservation 6(2): 93–107.
Ruiz-Mallen I., Dominguez P., Calvet-Mir L., Orta-Martinez M., Reyes-Garcia V. (2012). Applied research in ethnoecology: fieldwork experiences. Revista de Antropología Iberoamericana 7: 9–30. http://dx.doi.org/10.11156/aibr.070102e.
Russell C., Felker P. (1987). The Prickly-pears (Opuntia spp., Cactaceae): a source of human and animal food in semiarid regions. Economic Botany 41(3): 433–445. http://dx.doi.org/10.1007/BF02859062.
Shackleton S., Kirby D., Gambiza J. (2011). Invasive plants – friends or foes? Contribution of prickly pear (Opuntia ficus-indica) to livelihoods in Makana Municipality, Eastern Cape, South Africa. Development Southern Africa 28(2): 177–193. http://dx.doi.org/10.1080/0376835X.2011.570065.
Simenel R., Michon G., Auclair L., Thomas Y., Romagny B., Guyon M. (2009). Argan: the oil that hinders domestic forest. From product valorization to ecosystem naturalization. Autrepart (2): 51–73. http://dx.doi.org/10.3917/autr.050.0051. [In French].
Soliveres S., Eldridge D.J., Hemmings F., Maestre F.T. (2012). Nurse plant effects on plant species richness in drylands: the role of grazing, rainfall and species specificity. Perspectives in Plant Ecology, Evolution and Systematic 14(6): 402–410. http://dx.doi.org/10.1016/j.ppees.2012.09.003.
Takimoto A., Nair P.R., Nair V.D. (2008). Carbon stock and sequestration potential of traditional and improved agroforestry systems in the West African Sahel. Agriculture, Ecosystems & Environment 125(1): 159–166. http://dx.doi.org/10.1016/j.agee.2007.12.010.
Tasser E., Walde J., Tappeiner U., Teutsch A., Noggler W. (2007). Land-use changes and natural reforestation in the Eastern Central Alps. Agriculture, Ecosystems and Environment 118 (2007) 115–129. http://dx.doi.org/10.1016/j.agee.2006.05.004.
Tscharntke T., Klein A.M., Steffan-Dewenter I., Thies C. (2005). Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecology Letters 8: 857–874. http://dx.doi.org/10.1111/j.1461-0248.2005.00782.x.
Tucker C.J., Dregne H.E., Newcomb W.W. (1991). Expansion and contraction of the Sahara Desert. Science 253: 299–301. http://dx.doi.org/10.1126/science.253.5017.299.
Vásquez-Méndez R., Ventura-Ramos E., Oleschko K., Hernández-Sandoval L., Parrot J.F., Nearing M.A. (2010). Soil erosion and runoff in different vegetation patches from semiarid Central Mexico. Catena 80(3): 162–169. http://dx.doi.org/10.1016/j.catena.2009.11.003.
Vila M., Burriel J.A., Pino J., Chamizo J., Llach E., Porterias M., Vives M. (2003). Association between Opuntia species invasion and changes in land-cover in the Mediterranean region. Global Change Biology 9(8): 1234–1239. http://dx.doi.org/10.1046/j.1365-2486.2003.00652.x.
Wang X.M., Zhang C.X., Hasi E., Dong Z.B. (2010). Has the Three Norths Forest Shelterbelt program solved the desertification and dust storm problems in arid and semiarid China? Journal of Arid Environments 74(1): 13–22. http://dx.doi.org/10.1016/j.jaridenv.2009.08.001.
Wezel A., Rajot J.L., Herbrig C. (2000). Influence of shrubs on soil characteristics and their function in Sahelian agro-ecosystems in semi-arid Niger. Journal of arid environments 44(4): 383–398. http://dx.doi.org/10.1006/jare.1999.0609.
Whittaker R.H. (1975). Communities and ecosystems. McMillan Publ. Co, New York. 385 p.
Xu D., Li C., Song X., Ren H. (2014). The dynamics of desertification in the farming-pastoral region of North China over the past 10 years and their relationship to climate change and human activity. Catena 123: 11–22. http://dx.doi.org/10.1016/j.catena.2014.07.004.
Yous F.Z. (2016). Dynamique végétale et remontée biologique dans les plantations de Cactus du sud-ouest marocain (Ait Baamrane). Master Thesis, Cadi Ayyad University, Marrakech, Morocco. 66 p.
Zhao H.L., Zhou R.L., Su Y.Z., Zhang H., Zhao L.Y., Drake S. (2007). Shrub facilitation of desert land restoration in the Horqin Sand Land of Inner Mongolia. Ecological engineering 31(1): 1–8. http://dx.doi.org/10.1016/j.ecoleng.2007.04.010.
Zimmermann H.G., Moran V.C. (1991). Biological control of prickly pear, Opuntia ficus-indica, in South Africa. Agriculture, Ecosystems and Environment 37: 29–35. http://dx.doi.org/10.1016/0167-8809(91)90137-M.
Total of 76 references.

