Effect of photoperiod and fertilization on shoot and fine root growth in Picea abies seedlings
Fløistad I. S., Eldhuset T. D. (2017). Effect of photoperiod and fertilization on shoot and fine root growth in Picea abies seedlings. Silva Fennica vol. 51 no. 1 article id 1704. https://doi.org/10.14214/sf.1704
Highlights
- Effects of photoperiod and fertilization treatment on Norway spruce seedling growth were examined
- Short day treatment and ordinary K:N ratio in the fertilization proved the best combination for achieving seedlings with suitable root and shoot properties for field establishment
- Increased K:N ratio in the fertilization did not reduce shoot height growth.
Abstract
Picea abies seedlings were given three different fertilization treatments in the nutrient solution by varying the potassium:nitrogen (K:N) ratios (2.5, 3.0 or 3.9 g g–1). All fertilization treatments were combined with short-day (SD) treatment or no such treatment (control). Above- and belowground growth responses in the seedlings were analyzed. The SD treatment resulted in significantly reduced shoot height, compared to untreated control, irrespective of K:N ratio. No combination of photoperiod treatment or fertilization treatment affected the root collar diameter. In the current year root fraction with diameter < 0.5 mm, the highest K:N ratio led to significantly increased root length in control plants. In each 0.1 mm root diameter class up to 0.5 mm, the highest K:N ratio significantly stimulated root growth in control plants, while the effect was less evident for SD plants. SD treatment stimulated length growth in some fine root diameter classes. We conclude that SD treatment is a good and sufficient measure to reduce height growth without compromising fine root growth of P. abies seedlings. Fertilization treatment did not significantly improve aboveground growth in SD treated seedlings, and only limited effects on root growth was seen on control plants.
Keywords
Norway spruce;
nitrogen;
nutrients;
potassium;
root collar diameter;
root scanning;
short day treatment
Received 22 September 2016 Accepted 19 December 2016 Published 2 February 2017
Views 103562
Available at https://doi.org/10.14214/sf.1704 | Download PDF

1 Introduction
Sturdy seedlings with a high root growth capacity are needed to establish successful regeneration following clear-cutting and planting of Picea abies (L.) Karst. (Mattsson 1997; Simpson and Ritchie 1997; Grossnickle 2012). The nursery cultural practice may alter seedling attributes in several ways and thereby affect seedling performance (Mattsson 1997; Grossnickle 2012). Among material attributes seedling height, diameter and shoot:root weight ratio are all of importance. While seedlings’ shoot:root ratio is thought to influence their drought avoidance potential (Thompson 1985), root collar diameter influences the seedlings’ capability to avoid attack by pine weevils (Hylobius abietis L.) (Thorsén et al. 2001). When two-year old seedlings are produced in multipot containers the containers are moved from the greenhouse to outdoors for the first winter. During the second season seedlings grow in their multipot containers in an outdoor field. When spring starts early, and especially if spring is particularly warm, seedlings grown in a two year rotation could become too tall and obtain an unbalanced shoot:root ratio (Ministry of Agriculture 1996). This is often the case e.g. in Norwegian nurseries (Kohmann and Johnsen 2007).
Artificial shortening of photoperiod by short-day (SD) treatment in late summer is used regularly in boreal forest nurseries to induce height growth cessation and enhance frost hardiness development (Dormling et al. 1968; Colombo et al. 2001; Fløistad and Granhus 2013). Two to three weeks of SD treatment is a common routine in forest nurseries in Norway (Fløistad and Granhus 2013). However, it has also been shown that the duration of the SD treatment affects seedling diameter and the incidence of autumn shoots (Konttinen et al. 2003; Luoranen et al. 2009; Fløistad and Granhus 2013). In addition, both fertilization and day length during the SD treatment influence on the seedlings radial growth during and after SD treatment (Bjørnseth 1977; Colombo et al. 2001).
Plants that are grown with excess nitrogen usually have increased shoot growth and reduced root growth, with a high shoot:root ratio as a result (Salisbury and Ross 1992). For overcoming planting stress, however, root growth is crucial (Grossnickle 2005). Lamhamedi et al. (2013) showed that SD treatment of Picea mariana (Mill.) Britton, Sterns & Poggenb. seedlings led to increased root growth and better plug cohesion compared to control seedlings. They explained this by the earlier bud set of SD treated seedlings, followed by increased translocation of photosynthates to the root system. In accordance with this, Hawkins et al. (1994) found SD treatment to promote photosynthesis and thereby provide carbohydrates for increased root development. Jacobs et al. (2008) showed that SD treatment of Pseudotsuga menziesii (Mirb.) Franco seedlings (plug plants) led to increased root proliferation compared to control plants at 10 °C, while the opposite was the case at 20 °C. The root growth was evaluated periodically by root weight during a four month growth period in hydroponics without nutrient addition. Luoranen et al. (2007) found that SD treatment enhanced root egress (evaluated by weight) of summer-planted P. abies seedlings under dry conditions.
To produce sturdy seedlings with optimal root systems, it would be of considerable value for practical seedling production to determine if there is a possibility to alter fertilization routines within the frame of today’s routines in nurseries, without compromising other seedling quality traits. Then, timing and duration of SD treatment also could be optimized in order to avoid autumn bud break (Hawkins et al. 1996; Kohmann and Johnsen 2007; Luoranen et al. 2009). The fertilization routines in Norwegian nurseries are based on optimal nutrient concentrations in needles according to Ingestad (1979). Nitrogen (N) is used as one key indicator of the nutrient status. This element stimulates shoot height growth and competes with potassium (K) regarding uptake, so one possibility might be to manipulate the K:N ratio in the fertilizer by increasing the relative amount of K. This has been tried in commercial nurseries and we wanted to test the effect under more controlled conditions. Potassium is an element required for the functioning of many physiological and biochemical processes (Marschner 1986). Increased K addition in nutrient solution has been shown to increase root dry weight of birch seedlings (Ericsson and Kähr 1993), but in their work the root length or root collar diameter were not measured.
The aim of present study was to identify responses in P. abies seedlings after different combinations of photoperiod and fertilization. Our hypotheses were: (1) Increased K:N ratio in the nutrient solution from bud break in spring to terminal bud formation in autumn will reduce shoot growth and (2) Short day treatment will result in increased root weight and root length growth.
2 Materials and methods
2.1 Seedling material and experimental conditions
P. abies seeds, originating from Sanderud seed orchard (seed lot F06-037), were sown 5 June 2014 in limed peat, mixed with 25% perlite, in multipot containers (75 cm3 pots, 500 seedlings m–2, 60 pots per container). Germination and the first year growth phase took place in the greenhouse of the commercial forest nursery in Hokksund (59°46´N, 09°53´E), Buskerud County, Norway.
Prior to the experiment, on 30 April 2015, seedlings were brought to the experimental greenhouse of the Norwegian Institute of Bioeconomy Research at Hoxmark, Ås (59°40´N, 10°51´E). Seedlings were grown under natural light conditions with day temperatures varying between 20 and 30 °C. Bud break appeared shortly after moving the seedlings to the experimental greenhouse. Three to five times per week seedlings were watered and uniformly fertilized from below with a complete nutrient solution (Pioner 10-4-25 + Mikro). The compositions of the nutrient solutions are given in Table 1. Conductivity of the nutrient solution was 1.5 mS cm–1. Calcium nitrate (Ca(NO3)2) solution with N concentration 15.5% was added as an extra source of nitrogen to all seedlings by watering from above. The treatments consisted of three different K:N ratios in the experimental nutrient solutions combined with SD treatment or no such treatment (control). The different fertilization treatments started on 25 May. To provide the different levels of K:N, MKP (mono potassium phosphate) was added in the nutrient solution throughout the experimental period for treatments with the two highest K:N ratios (Table 1). From 11 August all seedlings were again given identical fertilization without MKP. Concentration of the nutrient solution was gradually reduced from that date by reduced conductivity of the nutrient solution to 1.0 and 0.7 mS cm–1 from mid-August and mid-September, respectively.
| Table 1. Compositions of the experimental nutrient solutions. Concentrations are given in mg l–1. | |||||||||||
| Element | N | P | K | Mg | S | Fe | Mn | Cu | B | Zn | Mo |
| K:N 2.5 (g:g) (Pioner 10-4-25 + mikro) | 12.4 | 5 | 31.6 | 5.5 | 7.1 | 0.3 | 0.09 | 0.03 | 0.04 | 0.05 | 0.01 |
| K:N 3.0 (g:g) (Pioner 10-4-25 + mikro) + MKP*) | 9.9 | 7.4 | 29.6 | 4.4 | 5.7 | 0.3 | 0.09 | 0.03 | 0.04 | 0.05 | 0.01 |
| K:N 3.9 (g:g) (Pioner 10-4-25 + mikro) + MKP*) | 9.9 | 14.2 | 38.2 | 4.4 | 5.7 | 0.3 | 0.09 | 0.03 | 0.04 | 0.05 | 0.01 |
| *) MKP – mono potassium phosphate | |||||||||||
SD treatment was performed during two weeks from 29 June to 13 July by covering seedlings with coextruded black and white polyethylene film from 19:00 to 09:00. At that time of the year, natural day length is about 18.5 hours.
2.2 Measurements and registration of bud set
Seedling height and root collar diameter were measured every month on 10 seedlings per treatment and replicate from the start of the experiment at 30 April. The same seedlings were measured each time. Seedling height was measured from the edge of the pots in the container to the terminal meristem of the seedlings (Mexal and Landis 1990) with an accuracy of 0.5 cm. Root collar diameter was measured concomitantly on the same seedlings with an accuracy of 0.01 mm. Shoots were harvested to analyze needle element concentrations at a commercial laboratory (Eurofins) accredited in accordance with DIN EN ISO/IEC 17025. The first harvest was on 28 May before start of the experimental fertilization treatment, the next harvest was performed when terminating the SD treatment at 13 July. The last shoot harvest on 20 October was after growth cessation in autumn. Nitrogen was analyzed by the Dumas method (VDLUFA Methodenbuch, Band II, 3.5.2.7). For P, K, Mg, Ca and S the material was digested with HNO3 in a microwave oven and analyzed by ICP-OES (DIN EN ESO 11885; 1998-04). From mid-July and onwards, terminal bud formation was recorded twice a week on all seedlings in one multipot container per treatment and replicate, until all seedlings had formed a tiny white terminal bud, corresponding to stage 1 on the scale of Johnsen (1989).
2.3 Root classification
At final harvest on October 20, the root system of eight plants per treatment and replicate were carefully washed over sieves with 2.0, 0.6 and 0.2 mm mesh screen (lowest mesh at the bottom) in order to remove soil without the loss of roots. Living current year roots, that is roots with white, flexible but not tough stele were cut off and stored in tap water at 4 °C until further processing after one to four weeks. Length per diameter fraction was determined using WinRhizo V2013a (Régent Instruments Canada Inc.) with scanner Epson Expression 11000XL (Epson America Inc.). Based on images created from the original digital root image, WinRhizo provides diameter distributions of the total root length by assigning root lengths to the diameter classes predefined by the user. We chose 0.1 mm diameter classes up to 1.0 mm, followed by 0.2 mm classes up to 2.0 mm and one class for diameters above 2.0 mm. Definition of root class: e.g. root class 0.1–0.2 mm means diameters from 0.1 mm up to, but not including, 0.2 mm. The five lowest diameter classes were called D1 (0–0.1 mm); D2 (0.1–0.2 mm); D3 (0.2–0.3 mm); D4 (0.3–0.4 mm); D5 (0.4–0.5 mm). Current year roots and previous year’s roots were then dried separately for two days at 70 °C and weighed. In the smallest diameter class there was probably a loss of several of the finest root fragments during the root cleaning process.
2.4 Experimental design and statistical analysis
The experiment was conducted in a greenhouse and the greenhouse tables were used as fertilization treatment units. SD treatment was performed on half of the seedlings on each greenhouse table. The experiment consisted of three multipot containers (60 pots per container) for each of the six treatments and four replicates, a total of 72 multipot containers in the experiment. Containers with P. abies seedlings were placed on the perimeter of the tables, but not included in the experiment.
The model for two-way ANOVA was:
![]()
where Yijk is the measured growth parameter or number of days to bud set for the ith photoperiod (i = 1,2) and the jth fertilization treatment (j = 1,2,3), µ is the overall mean, τi is the effect of photoperiod, δj is the effect of fertilization treatment, (τδ)ij is the interaction between photoperiod and fertilization treatment, ßk is the random effect of replicate, and εijk is the error term. ANOVA with comparison of least means squares by their contrasts and with a significance level of p = 0.05 was performed using the program JMP10 (SAS Institute 2007).
3 Results
Terminal bud set was ten days earlier in SD treated seedlings than in seedlings without such treatment, 33 and 43 days from start of treatment, respectively (Fig. 1A). No differences in terminal bud formation appeared within the different fertilization treatments. After the end of the SD treatment there was no further shoot height growth in the SD seedlings regardless of the fertilization treatment, while the control seedlings continued to grow for several weeks (Fig. 1A). This resulted in a significantly lower shoot height of all SD treated seedlings compared to control seedlings at the final measurement (p < 0.040). Within SD treatment, shoot height was not significantly affected by fertilization treatment. However, in control seedlings the mean value for shoot height was lower at the highest K:N ratio, but the difference was not significant (Fig. 1A). Neither SD treatment nor fertilization treatment had a significant effect on root collar diameter at the final measurements (Fig. 1B).
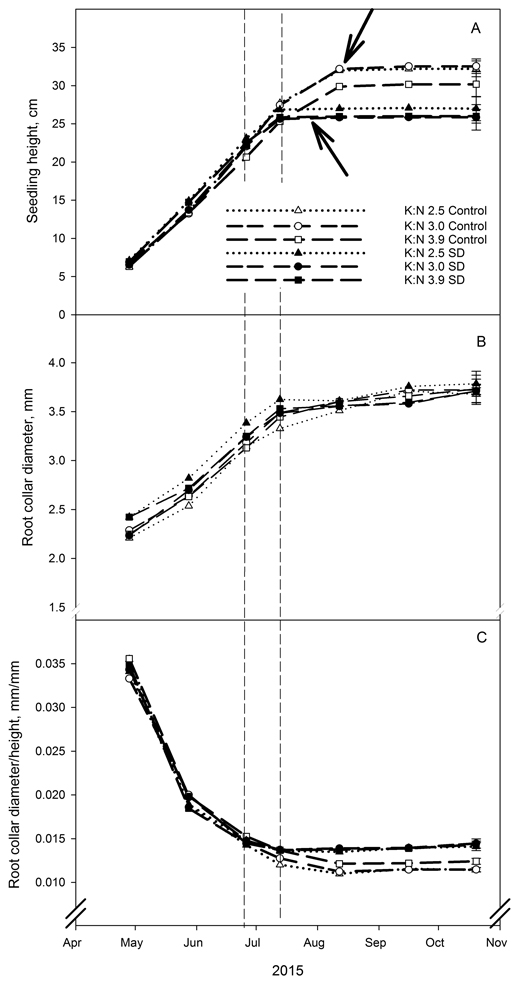
Fig. 1. Root collar diameter (mm) (A), shoot height (cm) (B) and ratio between root collar diameter and shoot height (mm/mm) (C) in Picea abies seedlings that had been given different combinations of photoperiod (SD treatment or control) and nutrients (three different K:N ratios in fertilization). K:N is the potassium:nitrogen (g:g) ratio in the nutrient solution. Broken vertical lines indicate the duration of the SD treatment. The variables were measured on the same ten plants per treatment and replicate at every measurement occasion. Vertical bars denote standard errors. Lower and upper arrows indicate bud formation in SD treated and control seedlings, respectively.
The SD treatment resulted in a significantly higher relationship between root collar diameter and shoot height (Fig. 1C; p < 0.003 at the final harvest). The reason for this was the reduced shoot height due to SD treatment. The fertilization treatment did not have any significant effect on the relationship between root collar diameter and height (Fig. 1C).
Fig. 2 shows that for pooled data on current year roots, more than 99% of the mean root length per plant belonged to the root diameter classes < 2.0 mm and thus per definition were fine roots. Out of these roots, 68% had diameter below 0.5 mm and thus per definition constituted the finest root fraction.
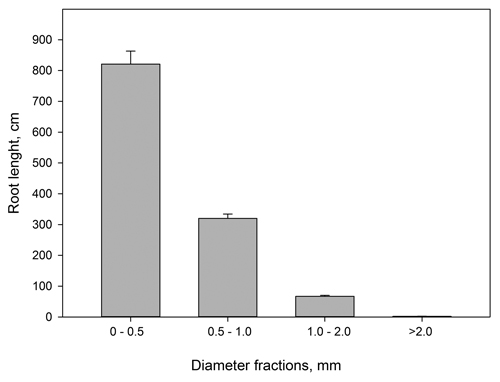
Fig. 2. Current year root lengths (cm) from Picea abies grouped in different diameter classes. n = 46–48. Vertical bars denote standard errors. The figure is based on pooled root length data.
Comparing sum root length of current year finest roots (diameters < 0.5 mm) showed that there was no significant effect of photoperiodic treatment on root length within each fertilization treatment (p > 0.063). However, for control plants the highest K:N ratio resulted in significantly higher root lengths (p < 0.018) than the lower K:N ratios (Table 2). No significant differences in sum root length appeared among different K:N ratios in SD treated seedlings (p > 0.22). The dry weight of the current year roots was not significantly affected (p > 0.087) by photoperiod. However, within each photoperiod treatment fertilization had an effect on current year root dry weight (Table 2). Within control seedlings, K:N ratio 3.0 in the fertilization resulted in significantly lower root dry weight than K:N ratio 2.5 and 3.9 (p < 0.037 and p < 0.016, respectively). For SD treated seedlings, K:N ratio 3.0 in the fertilization resulted in significantly lower root dry weight than K:N ratio 3.9 (p < 0.017).
| Table 2. Sum length of roots < 0.5 mm diameter, and dry weight of present year’s roots, from Picea abies seedlings that had received different combinations of photoperiod (SD treatment or control) and nutrients (three different K:N ratios in fertilization) (n = 8). K:N is the potassium:nitrogen (g:g) ratio in the nutrient solution. Different e letters notify significant effect (p < 0.05) within photoperiod treatment as determined by contrast analysis. The values denote mean value per seedling. | ||||||
| Fertilization treatment/ Photoperiod | K:N 2.5/ Control | K:N 3.0/ Control | K:N 3.9/ Control | K:N 2.5/ SD | K:N 3.0/ SD | K:N 3.9/ SD |
| Root length, cm | 661.7a | 590.1a | 979.8b | 860.0a | 838.0a | 998.8a |
| Dry weight, g | 0.606b | 0.460a | 0.624b | 0.657ab | 0.574a | 0.736b |
When dividing the current year finest roots in five diameter classes, results showed that SD treatment increased the root length significantly (p < 0.046) for some diameter classes and K:N ratios (Fig. 3). Root lengths increased with the highest K:N ratio compared to lower K:N ratios. This difference was significant for all diameter classes in control seedlings and for diameter classes D3 and D5 in SD seedlings (p < 0.026) (Fig. 4).
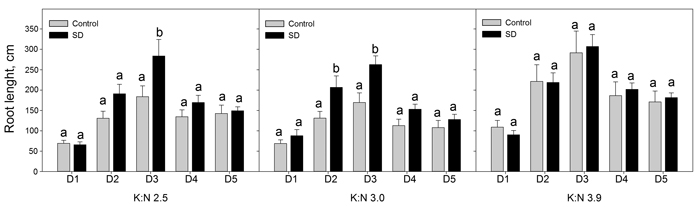
Fig. 3. Sum root lengths of current year roots in diameter classes D1 to D5. The seedlings had been given different combinations of photoperiod (SD treatment or control) and nutrients (three different K:N ratios in fertilization). K:N is the potassium:nitrogen (g:g) ratio in the nutrient solution. The diameter classes were (mm): D1 = 0–0.1; D2 = 0.1–0.2; D3 = 0.2–0.3; D4 = 0.3–0.4; D5 = 0.4–0.5. n = 8. Different letters notify significant effect (p < 0.05) of photoperiod within K:N level and root diameter class. Vertical bars denote standard errors. View larger in new window/tab.
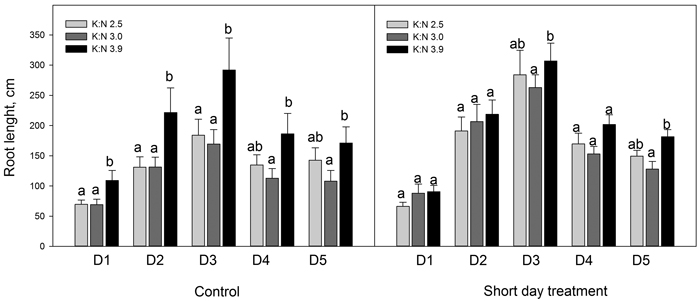
Fig. 4. Sum root lengths of current year roots in diameter classes D1 to D5. The seedlings had been given different combinations of photoperiod (SD treatment or control) and nutrients (three different K:N ratios in fertilization). K:N is the potassium:nitrogen (g:g) ratio in the nutrient solution. The root diameter classes were (mm): D1 = 0–0.1; D2 = 0.1–0.2; D3 = 0.2–0.3; D4 = 0.3–0.4; D5 = 0.4–0.5. n = 8. Different letters notify significant effect (p < 0.05) of K:N level within photoperiod and root diameter class. Vertical bars denote standard errors.
The concentrations of N, P, K, Mg, Ca and S in the needles at each harvest are shown in Fig. 5. The figure is based on pooled data because the concentrations of each element were not significantly different from each other among treatments within each date (p < 0.05). At the final harvest the mean element concentrations in needles were as follows in % of dry matter: N 1.7, P 0.29, K 1.7, Mg 0.12, Ca 0.29 and S 0.18 (Fig. 5). At the second and third harvest, the K concentration in the needles was about twice as high as at the first harvest, irrespective of treatment.
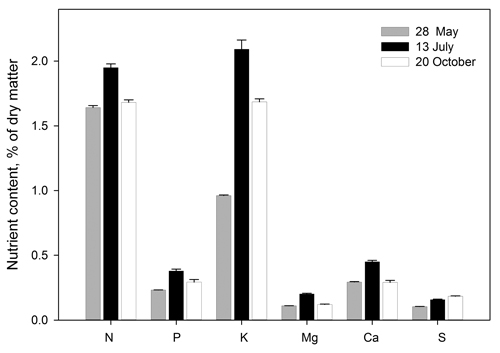
Fig. 5. Element concentrations in Picea abies needles before start of the experimental fertilization treatment (28.05), when terminating the SD treatment (13.07) and following growth cessation in autumn (20.10). The figure is based on pooled data (all 24 observations at each date, i.e. four replications per treatment) because the concentrations of each element were not significantly different from each other among treatments within each date (p < 0.05). Bars denote standard errors.
4 Discussion
In accordance with what is previously well documented (Dormling et al. 1968; Heide 1974; Fløistad and Granhus 2013) we found a significant effect of SD treatment on shoot height. Root collar diameter was not influenced by SD treatment in our experiment, in contrast to what is previously found (Konttinen et al. 2007; Fløistad and Granhus 2013). Both temperature and nutrition have been reported to impact on root collar diameter in P. abies (Bjørnseth 1977; Fløistad and Granhus 2010). The nutrient level in our seedlings may be one possible explanation of the lack of differences (Bjørnseth 1977; Ingestad 1979) even if none of the fertilization treatments influenced on shoot height growth. Photoperiod governed shoot height to a much higher extent than nutrition did in our experiment. Therefore, our first hypothesis that increased K:N ratio in the nutrient solution could reduce shoot growth was not confirmed. If lowering the seedlings’ access to nutrients, seedling growth will be affected (Grossnickle 2000; Fløistad and Kohmann 2004). This was not the case in our experiment. Possibly, at a K:N ratio high enough to affect height growth in P. abies seedlings, the nitrogen level would have become too low to produce high quality seedlings for reforestation.
Our second hypothesis was partly verified. By scanning root lengths per diameter class up to 0.5 mm we showed significant increased root length in some of the diameter classes following SD treatment or K:N ratio of 3.9, respectively. To our knowledge, root growth evaluation by root lengths of different diameter classes has not been performed in this type of experiment before. Dry weight determination showed significantly lowest dry weight with K:N ratio 3.0 in the fertilization. The finest roots are the most important for water and nutrient uptake. Thus, it is important to be able to assess differences in the length growth of the finest roots and rely not only on the less specific weight measurements.
After bud set, resources are allocated to storage, diameter growth and root growth, as shown in several species (Cannell and Willett 1976; Friend et al. 2013; McKown et al. 2016). It is known that P. abies in the field in the temperate zone has a new flush of root growth early in the autumn (Lyr and Hoffmann 1967; Puhe 2003). Because the SD plants had ten days earlier bud set than control seedlings, they may have started to allocate carbohydrates to root growth earlier than the control plants did (Lamhamedi et al. 2013). Possibly, this is one reason for the better shoot growth that has often been observed after planting of SD treated plants compared to control plants (Hawkins et al. 1996; Rostad et al. 2006). However, there is a complex interaction between many factors to regulate the shoot: root ratio, including onset and length of SD treatment, temperature and soil moisture (e.g. Luoranen et al. 2007; Jacobs et al. 2008; Fløistad and Granhus 2013). These factors were not accounted for in our study. The positive effect of increased K addition on root length growth in some diameter classes, most evident in control plants, remains an unexplained phenomenon.
At the second and the third (final) harvest, needle K concentration was almost doubled compared to the situation at the start, irrespective of the level of K:N ratio in the nutrient solution. In all treatments, the nitrogen concentration in the needles at the final harvest was somewhat lower (1.7%) than the concentrations typically measured in needles from commercial forest nurseries (1.8–2.2%). The seedlings would probably have benefited from a more frequent N addition. It seems that the uptake of K may have been high at the expense of N uptake. Even in forest nurseries, plants with a low N concentration often have a high K concentration. This may be due to luxury consumption of K at reduced N levels, as described by Ingestad and Kähr (1985) and also supported by the results of Fløistad and Kohmann (2004). The regulation of cation uptake in Norway spruce is found to be less efficient than for Scots pine (Ingestad 1979). This may explain the high K concentration found in the needles in our experiment, and also the somewhat high Ca concentration due to uptake from the Ca(NO3)2 solution given as extra N source. The needle concentrations of P, Mg and S were close to those reported in Norway spruce seedlings by Ingestad (1979) and similar to typical values from Norwegian nurseries (Fløistad, unreported data).
We conclude that, in addition to the well-known reduction in shoot height growth due to SD treatment, our results indicated that this treatment had a positive effect on length growth of the finest roots. This was evident only for some of the root diameter classes. Addition of extra K did not significantly improve above- or belowground growth of plants after SD treatment and did not have a convincing positive effect on control plants. We expect that the better fine root growth after SD treatment will improve the establishment ability of the plants in the field.
Acknowledgements
We would like to thank Anne E. Nilsen, Helge Meissner, Geir Østreng and Marit Helgheim for valuable technical assistance, and Dr. Therese With Berge for lending us the WinRhizo V2013 equipment. Thanks are due to the Norwegian Development Fund for Forestry, the Norwegian Forestry Foundation “Skogtiltaksfondet”, Borregaard Research Fund and Norwegian Institute of Bioeconomy Research for financing.
References
Bjørnseth I.-P. (1977). Cessation of cambial activity in Norway spruce (Picea abies (L.) Karst.), its relation to natural daylength, temperature and nutrition. Department of Forest Genetics, Royal College of Forestry, Stockholm, Sweden. Research Notes 27: 32–39.
Cannell M.G.R., Willett S.C. (1976). Shoot growth phenology, dry matter distribution and root: shoot ratios of provenances of Populus trichocarpa, Picea sitchensis and Pinus contorta growing in Scotland. Silva Genetica 25: 49–59.
Colombo S.J., Menzies M.I., O’Reilly C. (2001). Influence of nursery cultural practices on cold hardiness of coniferous forest tree seedlings. In: Bigras F.J., Colombo S.J. (eds.). Conifer cold hardiness. Vol. 1 of the series Tree Physiology. Kluwer Academic Publishers. p. 223–252. https://doi.org/10.1007/978-94-015-9650-3_9.
Dormling I., Gustafsson Å., von Wettstein D. (1968). The experimental control of the life cycle in Picea abies (L.) Karst. I. Some basic experiments on the vegetative cycle. Silvae Genetica 17: 44–64.
Ericsson T., Kähr M. (1993). Growth and nutrition in birch seedlings in relation to potassium supply rate. Trees 7(2): 78–85. https://doi.org/10.1007/BF00225473.
Fløistad I.S., Granhus A. (2010). Bud break and spring frost hardiness in Picea abies seedlings in response to photoperiod and temperature treatments. Canadian Journal of Forest Research 40(5): 968–976. https://doi.org/10.1139/X10-050.
Fløistad I.S., Granhus A. (2013). Timing and duration of short-day treatment influence morphology and second bud flush in Picea abies seedlings. Silva Fennica 47(3) article 1009. https://doi.org/10.14214/sf.1009.
Fløistad I.S., Kohmann K. (2004). Influence of nutrient supply on spring frost hardiness and time of bud break in Norway spruce (Picea abies (L.) Karst.) seedlings. New Forests 27(1): 1–11. https://doi.org/10.1023/A:1025085403026.
Friend A.L., Coleman M.D., Isebrands J.G. (2013). Carbon allocation to root and shoot systems of woody plants. In: Davis D.T., Haissig B.E. (eds.). Biology of adventitious root formation. Springer Science & Business Media. p. 245–273.
Grossnickle S.C. (2000). Ecophysiology of northern spruce species. The performance of planted seedlings. NRC Research Press, Ottawa.
Grossnickle S.C. (2005). Importance of root growth in overcoming planting stress. New Forests 30(2): 273–294. https://doi.org/10.1007/s11056-004-8303-2.
Grossnickle S.C. (2012). Why seedligs survive: influence of plant attributes. New Forests 43(5): 711–738. https://doi.org/10.1007/s11056-012-9336-6.
Hawkins C.D.B., Eng R.Y.N., Krasowski M.J. (1994). Short day nursery treatment promotes photosynthesis in interior spruce seedlings: summary of poster. General Technical Report RM-GTR-257. p. 268–270.
Hawkins C.D.B., Eastham A.M., Story T.L., Eng R.Y.N., Draper D.A. (1996). The effect of nursery blackout application on Sitka spruce seedlings. Canadian Journal of Forest Research 26(12): 2201–2213. https://doi.org/10.1139/x26-249.
Heide O.M. (1974). Growth and dormancy in Norway spruce ecotypes (Picea abies). I. Interaction of photoperiod and temperature. Physiologia Plantarum 30(1): 1–12. https://doi.org/10.1111/j.1399-3054.1974.tb04983.x.
Ingestad T. (1979). Mineral nutrient requirements of Pinus silvestris and Picea abies seedlings. Physiologia Plantarum 45(4): 373–380. https://doi.org/10.1111/j.1399-3054.1979.tb02599.x.
Ingestad T., Kähr M. (1985). Nutrition and growth of coniferous seedlings at varied relative nitrogen addition rate. Physiologia Plantarum 65(2): 109–116. https://doi.org/10.1111/j.1399-3054.1985.tb02368.x.
Jacobs D.F., Davis A.S., Wilson B.C., Dumroese R.K., Goodman R.C., Salifu K.F. (2008). Short-day treatment alters Douglas-fir seedling dehardening and transplant root proliferation at varying rhizosphere temperatures. Canadian Journal of Forest Research 38(6): 1526–1535. https://doi.org/10.1139/x08-020.
Johnsen Ø. (1989). Freeze-testing young Picea abies plants. A methodological study. Scandinavian Journal of Forest Research 4(1–4): 351–367. https://doi.org/10.1080/02827588909382572.
Kohmann K., Johnsen Ø. (2007). Effects of early long-night treatment on diameter and height growth, second flush and frost tolerance in two-year-old Picea abies container seedlings. Scandinavian Journal of Forest Research 22(5): 375–383. https://doi.org/10.1080/02827580701520486.
Konttinen K., Rikala R., Luoranen J. (2003). Timing and duration of short-day treatment of Picea abies seedlings. Baltic Forestry 9: 2–9.
Konttinen K., Luoranen J., Rikala R. (2007). Growth and frost hardening of Picea abies seedlings after various night length treatments. Baltic Forestry 13: 140–148.
Lamhamedi M.S., Renaud M., Desjardins P., Veilleux L. (2013). Root growth, plug cohesion, mineral nutrition, and carbohydrate content of 1+0 Picea mariana seedlings in response to a short-day treatment. Tree Planters Notes 56: 35–46.
Luoranen J., Konttinen K., Rikala R. (2009). Frost hardening and risk of a second flush in Norway spruce seedlings after an early-season short-day treatment. Silva Fennica 43(2): 235–247. https://doi.org/10.14214/sf.209.
Lyr H., Hoffmann G. (1967). Growth rates and growth periodicity of tree roots. International Review of Forestry Research 2: 181–236. https://doi.org/10.1016/B978-1-4831-9976-4.50011-X.
Marschner H. (1986). Mineral nutrition of higher plants. Academic Press, London.
Mattsson A. (1997). Predicting field performance using seedling quality assessment. New Forests 13(1): 227–252. https://doi.org/10.1023/A:1006590409595.
McKown A.D., Guy R.D., Quamme L.K. (2016). Impacts of bud set and lammas phenology on root:shoot biomass partitioning and carbon gain physiology in poplar. Trees 30(6): 2131–2141. https://doi.org/10.1007/s00468-016-1439-9.
Mexal J.G., Landis T.D. (1990). Target seedling concepts: height and diameter. In: Rose R., Campbell S.J., Landis T.D. (eds.). Proceedings, Western Forest Nursery Association, 1990 August 13–17, Roseburg, OR. USDA Rocky Mountain Forest and Range Experiment Station, General Technical Report RM-200. p. 17–35.
Ministry of Agriculture (1996). Forskrift om skogfrø og skogplanter. Oslo, Norway. 5 p.
Puhe J. (2003). Growth and development of the root system of Norway spruce (Picea abies) in forest stands – a review. Forest Ecology and Management 175(1–3): 253–273. https://doi.org/10.1016/S0378-1127(02)00134-2.
Rostad H., Granhus A., Fløistad I.S., Morgenlie S. (2006). Early summer frost hardiness in Picea abies seedlings in response to photoperiodic treatment. Canadian Journal of Forest Research 36(11): 2966–2973. https://doi.org/10.1139/X06-167.
Salisbury F.B., Ross C.W. (1992). Plant physiology. Wadsworth Publishing Company, Belmont, California.
Simpson D.G., Ritchie G.A. (1997). Does RGP predict field performance? A debate. New Forest 13(1): 253–277. https://doi.org/10.1023/A:1006542526433.
Thompson B.E. (1985). Seedling morphological evaluation – what you can tell by looking. In: Duryea M.L. (ed.). Evaluating seedling quality: principles, procedures and abilities of major tests. Proceedings of the workshop held October 16–18, 1984. Forest Research Laboratory, Oregon State University, Corvallis. p. 59–71.
Thorsén Å., Mattsson S., Weslien J. (2001). Influence of stem diameter on the survival and growth of containerized Norway spruce seedlings attacked by pine weevils (Hylobius spp.). Scandinavian Journal of Forest Research 16(1): 54–66. https://doi.org/10.1080/028275801300004415.
Total of 36 references.


