The effects of drainage and restoration of pine mires on habitat structure, vegetation and ants
Punttila P., Autio O., Kotiaho J. S., Kotze D. J., Loukola O. J., Noreika N., Vuori A., Vepsäläinen K. (2016). The effects of drainage and restoration of pine mires on habitat structure, vegetation and ants. Silva Fennica vol. 50 no. 2 article id 1462. https://doi.org/10.14214/sf.1462
Highlights
- Mire drainage shifted floristic composition and ant assemblages towards forest communities
- Raising the water-table level by ditch filling and the thinning of trees affected mire communities positively already 1–3 years after the start of restoration
- The extent of tree cover, the coverage of Sphagnum mosses and the water-table level were major determinants of ant assemblage structure.
Abstract
Habitat loss and degradation are the main threats to biodiversity worldwide. For example, nearly 80% of peatlands in southern Finland have been drained. There is thus a need to safeguard the remaining pristine mires and to restore degraded ones. Ants play a pivotal role in many ecosystems and like many keystone plant species, shape ecosystem conditions for other biota. The effects of mire restoration and subsequent vegetation succession on ants, however, are poorly understood. We inventoried tree stands, vegetation, water-table level, and ants (with pitfall traps) in nine mires in southern Finland to explore differences in habitats, vegetation and ant assemblages among pristine, drained (30–40 years ago) and recently restored (1–3 years ago) pine mires. We expected that restoring the water-table level by ditch filling and reconstructing sparse tree stands by cuttings will recover mire vegetation and ants. We found predictable responses in habitat structure, floristic composition and ant assemblage structure both to drainage and restoration. However, for mire-specialist ants the results were variable and longer-term monitoring is needed to confirm the success of restoration since these social insects establish perennial colonies with long colony cycles. We conclude that restoring the water-table level and tree stand structure seem to recover the characteristic vegetation and ant assemblages in the short term. This recovery was likely enhanced because drained mires still had both acrotelm and catotelm, and connectedness was still reasonable for mire organisms to recolonize the restored mires either from local refugia or from populations of nearby mires.
Keywords
ecological restoration;
ditching;
Aichi Biodiversity Target 15;
Formicidae;
pine bogs and fens;
transforming and transformed drained mires;
water-table level
-
Punttila,
Finnish Environment Institute, P.O. Box 140, FI-00251 Helsinki, Finland
E-mail
pekka.punttila@ymparisto.fi

- Autio, Centre for Economic Development, Transport and the Environment in South Ostrobothnia, P.O. Box 252, FI-65101 Vaasa, Finland E-mail olli.autio@ely-keskus.fi
- Kotiaho, University of Jyväskylä, Department of Biology & Environmental Sciences, P.O. Box 35, FI-40014 Jyväskylä, Finland E-mail janne.kotiaho@jyu.fi
- Kotze, University of Helsinki, Department of Environmental Sciences, P.O. Box 65, FI-00014, University of Helsinki, Finland E-mail johan.kotze@helsinki.fi
- Loukola, University of Oulu, Department of Biology, P.O. Box 3000, FI-90014 Oulu, Finland E-mail olli.loukola@gmail.com
- Noreika, University of Helsinki, Department of Environmental Sciences, P.O. Box 65, FI-00014, University of Helsinki, Finland; University of Helsinki, Department of Biosciences, P.O. Box 65, FI-00014 University of Helsinki, Finland E-mail norbertas.noreika@gmail.com
- Vuori, University of Jyväskylä, Department of Biology & Environmental Sciences, P.O. Box 35, FI-40014 Jyväskylä, Finland E-mail anna@kureniemi.fi
- Vepsäläinen, University of Helsinki, Department of Biosciences, P.O. Box 65, FI-00014 University of Helsinki, Finland E-mail kari.vepsalainen@helsinki.fi
Received 7 September 2015 Accepted 16 December 2015 Published 19 January 2016
Views 345669
Available at https://doi.org/10.14214/sf.1462 | Download PDF

Supplementary Files
1 Introduction
The Global Peatland Database of the International Mire Conservation Group (IMCG) has estimated that peatlands represent about 3% of the globe’s total land mass, and that at least 80% of peatlands are located in areas with northern temperate or cold climates (Rydin and Jeglum 2006). Much of the original peatland area has already been lost (Rydin and Jeglum 2006). In Finland, peatlands cover 28% of the land mass, which is the largest share of peatlands globally (Auvinen et al. 2007). At present, of the 8.8 million ha of peatland about 4.1 million ha remain undrained and 4.6 million ha are drained (Peltola 2014).
Most of the Finnish pristine mires are situated in Lapland, whereas in southern Finland nearly 80% of peatlands have been drained (Auvinen et al. 2007). Drainage for forestry and agriculture, and peat harvesting are the main threats to the mire biota and habitat types in Finland (Kaakinen et al. 2008; Rassi et al. 2010; Working Group on a National Strategy for Mires and Peatlands 2011). Owing to drainage, the size and connectivity of mires have drastically decreased in southern Finland, which has impaired the movement and colonization of mire biota among habitats (Auvinen et al. 2010). The number of red-listed species living primarily and secondarily in Finnish mires is 223 and 197, respectively, comprising 8.5% of all red-listed species (Rassi et al. 2010). Because populations respond slowly to increased habitat loss at large spatial scales (Hanski 2008), many mire species are likely experiencing an extinction debt (Tilman et al. 1994; Hanski and Ovaskainen 2002). Furthermore, a number of mire-associated insects are habitat specialists (Spitzer and Danks 2006) and thus prone to extinction (Dunn 2005). An analysis of changes of red-list categories (i.e., degree of threat) of species in Finland between 2000 (Rassi et al. 2001) and 2010 (Rassi et al. 2010) showed that 30 mire-associated species have become more threatened, but only 4 species less threatened (Rassi et al. 2010). Also, populations of many mire bird and butterfly species have declined, which indicates an increasing number of threatened mire species (Auvinen et al. 2010).
Clearly, there is a need to safeguard the remaining pristine sites and to restore degraded ecosystems of European mires, and EU has agreed of the goal of restoring 15% of degraded ecosystems by 2020 (see the Aichi Biodiversity Target 15, CBD 2010; European Commission 2011). This target, however, seems unrealistic: heavy restoration measures must be completed across large areas and in a short time, while compensating for ongoing degradation elsewhere (Kotiaho et al. 2015; see also Kotiaho and Moilanen 2015).
Restoration is a process where the functions, structures, processes, and biotic communities of habitats and ecosystems are returned towards their pristine state (Society for Ecological Restoration International Science & Policy Working Group 2004; Rydin and Jeglum 2006) or turned into a desired state. Peatland restoration aims for the recovery of ecosystem functions: to return naturally functioning and self-sustaining, carbon-accumulating and nutrient-retaining mire ecosystems (Kuuluvainen et al. 2002; Vasander et al. 2003; Aapala et al. 2008). Important goals include the recovery of ecohydrological properties (the quantity and quality of in-flowing and out-flowing waters and naturally-fluctuating water-table levels, i.e. natural hydrology), the recovery of peat-forming vegetation (e.g. Sphagnum mosses) and the recovery of structural characteristics (e.g. species composition) and processes (e.g. succession) of the mire biota (Aapala et al. 2008). Many mires, even within the current conservation-area network of Finland, have been drained for forestry prior to the establishment of conservation areas. In 1989–2013 about 20 000 ha of these peatlands have already been restored and about 18 000 ha still need to be restored to safeguard the ecological value of the protected areas (Similä et al. 2014).
The effects of peatland restoration on the biota need further investigation, especially on habitat specialists (Rydin and Jeglum 2006). Ants provide valuable information when evaluating land-management actions and assessing long-term ecosystem changes because they are sensitive to changes in habitat quality and play a pivotal role in many ecosystems (Andersen and Majer 2004; Underwood and Fisher 2006; Fagan et al. 2010). They, like many keystone plant species, shape ecosystems for other organisms. The activities of ants and their nest and trail constructions may affect other biota considerably (Hölldobler and Wilson 1990; Punttila and Kilpeläinen 2009; Finér et al. 2013, and references therein).
Mires comprise the primary or secondary habitat for at least a third of the 55 native ant species of Finland (Punttila et al. 2013). In boreal areas, a few studies have shown that drainage of mires affects mire-ant species negatively (Krogerus 1960; Collingwood 1963; 1999; Vepsäläinen et al. 2000; Punttila and Kilpeläinen 2009), but knowledge on mire ant communities and their assembly processes are scarce globally (Sveum 1978; Vepsäläinen et al. 2000; Dlussky 2001; Ellison et al. 2002; Gotelli and Ellison 2002a; b; Mabelis and Chardon 2005; Ratchford et al. 2005; Sanders et al. 2007; Bujan et al. 2010). The effects of mire restoration and subsequent vegetation succession on ants and other insect fauna are poorly understood (Laiho et al. 2001; van Duinen et al. 2003; Watts et al. 2008; Elo et al. 2015; Noreika et al. 2015).
Our primary aim is to characterize differences in mire habitats, ant assemblages and the occurrence of individual ant species among pristine, drained and recently restored mires. We focused on three questions in the short term: (1) What are the most essential differences in vegetation between pristine and drained mires, and how do restoration affect these?; (2) Does the ant-assemblage structure differ between pristine and drained mires and how does restoration affect ant assemblages?; (3) How are the ant species distributed among pristine, drained and restored mires, and what are the most important mire characteristics affecting the ants? Further, our study serves as a baseline for future monitoring of the studied mires when longer-term restoration success is evaluated.
Generally, we test if restoring the water-table level by ditch filling and reconstructing naturally sparse and low pine stands by heavy thinning and partial clear-cutting, will restore habitats to allow recolonization and recovery of the vegetation and ants of pristine mire habitats.
2 Materials and methods
2.1 Study design
Metsähallitus Parks & Wildlife Finland manages the Finnish conservation area network and aims to restore mire ecosystems that have been drained for forestry prior to the establishment of conservation areas. Restoration success is also monitored (Aapala et al. 2012). We utilized a sampling-plot network established to monitor restoration success of drained pine mires with the goal of returning the drained mires to their natural state.
The nine study mires (Table 1) are located in two regions in Finland, Northern Karelia and Central Finland, along the border between the southern and middle boreal forest vegetation zones (Ahti et al. 1968), mostly in the eccentric bog zone (Sphagnum fuscum raised bogs) and partly (Kiemanneva and Väljänneva in Table 1) in the southern aapa mire zone (Ruuhijärvi 1983). The mires are mainly ombrotrophic, though oligotrophy is also wide-ranging and some mire parts are mesotrophic. All mires belong partly to the Natura 2000 network of nature protection areas but parts of the mires had been drained in 1960s–1970s prior to the establishment of nature conservation areas (Uusitalo et al. 2006). Restoration of parts of all the studied mires started in 2003–2005 by filling the ditches and by harvesting a varying proportion of trees in 2003–2006. During harvesting, timber and pulpwood were removed, but all tree stumps and varying amounts of logging residues (branches and tree tops) were left behind.
| Table 1. Mire types of the pristine, drained and restored treatments prior to the start of restoration in 2003, in the nine study mires in Central Finland and Northern Karelia, and their approximate coordinates. N = number of sampling locations representing the given mire type (total = 162 sampling locations). | |||
| Region/mire | Treatment | Mire types1 (N) | Coordinates |
| Central Finland | |||
| Kiemanneva | Pristine | IR (1), RiNR (3), TR (2) | 63°23´N, 25°16´E |
| Drained | muIR (3), TKg (3) | ||
| Restored | muIR (3), muRaR(3) | ||
| Väljänneva | Pristine | LkN (2), RaR(1), SR (3) | 63°19´N, 25°18´E |
| Drained | muIR (1), muRaR (3), muTR (1), TKg (1) | ||
| Restored | muIR (1), muKeR (3), muRaR (2) | ||
| Southern Kulhanvuori | Pristine | IR (1), SR (1), TR (4) | 62°34´N, 24°57´E |
| Drained | muIR (1), TKg (5) | ||
| Restored | muIR (3), TKg (3) | ||
| Northern Kulhanvuori | Pristine | LkR (6) | 62°35´N, 24°57´E |
| Drained | muRaR (1), TKg (5) | ||
| Restored | muRaR (1), TKg (5) | ||
| Northern Karelia | |||
| Ristisuo | Pristine | LkR (1), RaR (5) | 62°56´N, 31°20´E |
| Drained | muIR (3), muKgR (1), muRaR (2) | ||
| Restored | muIR (4), muPsR (1), muRaR (1) | ||
| Juurikkasuo | Pristine | LkR (1), RaR (5) | 62°56´N, 31°26´E |
| Drained | muIR (2), muKgR (1), TKg (2), VT (1) | ||
| Restored | muPsR (1), TKg (5) | ||
| Rapalahdensuo | Pristine | LkR (4), RaR (2) | 62°54´N, 29°30´E |
| Drained | muIR (2), muLkR (1), muRaR (1), TKg (2) | ||
| Restored | muIR (4), TKg (2) | ||
| Tiaissuo | Pristine | LkR (3), RaR (3) | 62°56´N, 29°24´E |
| Drained | muIR (4), TKg (2) | ||
| Restored | muIR (4), muRaR (2) | ||
| Heinäsuo | Pristine | LkN (1), RaR (5) | 62°54´N, 31°28´E |
| Restored-a | muIR (3), muLkR (2), muRaR (1) | ||
| Restored-b | LkR (1), muIR (4), muLkR (1) | ||
| 1 Mire type abbreviations are according to Eurola et al. (1995), and English translations are according to Raunio et al. (2008): IR = Dwarf shrub pine bogs, LkN = Low-sedge bogs & fens, LkR = Low-sedge pine fens, muIR = Transforming Dwarf shrub pine bogs, muKeR = Transforming Ridge-hollow pine bogs, muKgR = Transforming Thin-peated pine mires, muLkR = Transforming Low-sedge pine fens, muPsR = Transforming Carex globularis pine mires, muRaR = Transforming Sphagnum fuscum bogs, muTR = Transforming Eriophorum vaginatum pine bogs, RiNR = Flark pine fens, RaR = Sphagnum fuscum bogs, SR = Tall-sedge pine fens, TKg = Transformed drained mires, TR = Eriophorum vaginatum pine bogs, VT = Sub-xeric heath forests. | |||
For each mire, prior to the start of the restoration process in 2003, three separate study areas were selected to represent different treatments: (1) pristine mire, (2) drained mire and (3) restored mire (Table 1). In one of the mires (Heinäsuo), however, the area designated to remain drained was also restored and this mire thus provided one pristine and two restored areas but no drained area (Table 1). For each treatment per mire, two 250 m transects, on average 80 m apart, were established with three sampling locations per transect. This resulted in six sampling locations per treatment per mire (nine mires × three treatments × two transects × three sampling locations = 162 sampling locations). The treatments per mire were, on average, 600 m apart.
2.2 Vegetation, drainage and ant data collection
At each sampling location, the mire-site type was recorded prior to restoration measures in 2003 (Table 1), and several sets of data were collected: (1) tree-stand characteristics were recorded from a circular 100 m2 tree-sampling plot, in the middle of which (2) tree-sapling and microsite-type data were recorded from a 25 m2 sapling square. In each corner of the 25 m2 sapling square, (3) the covers of vascular plant, moss and lichen species, and litter and surface water were estimated within 1 m2 vegetation squares (four squares per sapling square). The coverages were estimated on a scale 0.1, 0.5, 1, 3, 5, 7, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 95, 97, 100%. Data sets 1–3 above were collected both prior to restoration in 2003, and again in 2007, when we sampled the ants. Here we mainly use the 2007 data to characterize sampling locations and to provide environmental variables for the ant analyses. Additionally, in 2007, (4) the water-table level was monitored using plastic tubes (PVC pipes, length 88 cm, diameter 20 mm) as ground water wells. The tubes were set in two opposite corners of each 25 m2 sapling square. Water-table depth, i.e. distance of the water table from the mire surface, was measured with an accuracy of 1 cm at two-week intervals, six times from early June to mid-August in 2007.
Three circular tree-sampling plots (radius 5.64 m = 100 m2) were evenly placed along each transect, approximately 62.5 m apart. From each tree-sampling plot, tree (height > 150 cm) species and their stem numbers were recorded. The trees were classified into size classes according to their diameter at breast height (DBH, d1.3 m classes < 7, 7–20 and > 20 cm) and height (classes 1.5–3, 3–8 and > 8 m). Dead standing trees (snags) and fallen dead trees (logs) were recorded in a similar way as the living trees (at 1.3 m DBH from the ground for snags and 1.3 m from the butt end for logs).
Tree-sapling and bush (height 50–150 cm) species and their numbers were recorded from the sapling square (25 m2) within the tree-sampling plot. Within the sapling square the percentage covers of mire surface topography types, i.e. microsite types (hummock, lawn and flark) were estimated to the nearest 10 percent.
Based on a-priori knowledge of the general importance of different environmental characteristics on the occurrence of ants, we selected a set of potential explanatory variables in the analyses on ants. Variables selected relate to the degree of shading of canopy layers of different height and vegetation type, to the occurrence of potential nesting sites, and to the occurrence of potential prey and aphid colonies. Also, the most important predictor variables indicating successful restoration were included. The variables used included (1) treatment (a three-level factor; pristine, drained, restored), and several variables from (2) the 100 m2 tree-sampling plots (number of low trees [1.5–3 m], tall trees [> 3m] and dead trees), (3) the 25 m2 sapling squares (number of tree saplings and proportions of the microsite types hummock, lawn and flark), (4) the 1 m2 vegetation squares (percentage covers of surface water and litter, the pooled cover of Sphagnum spp., the pooled cover of other mosses, the pooled cover of herbs, sedges and grasses (i.e. non-woody annual plants), the pooled cover of low (< 20 cm) dwarf shrubs, and the pooled cover of tall (> 20 cm) dwarf shrubs and shrubs (for details, see below); average cover of the four 1 m2 vegetation squares was used) and (5) water-table depth (average of the two wells per sapling square).
Vegetation data were used to characterize the microhabitat types of the sampling locations such that they would reflect the main ecological gradients among the sampling locations. We calculated the combined cover of (1) Sphagnum spp. mosses, (2) other mosses, (3) herbs, sedges and grasses (sensu Eurola et al. 1995) that do not provide shade in early spring and late autumn, (4) low dwarf shrubs (species typically < 20 cm in height, Andromeda polifolia L., Calluna vulgaris (L.) Hull, Empetrum nigrum L., Vaccinium myrtillus L., V. vitis-idaea L.) excluding the recumbent V. oxycoccos L. and V. microcarpum (Turcz. ex Rupr.) Schmalh., and (5) tall dwarf shrubs (Chamaedaphne calyculata (L.) Moench, Ledum palustre L., V. uliginosum L.) pooled with shrubs (Betula nana L., Salix myrsinifolia Salisb.) that provide shade throughout the growing season.
Ants were sampled using pitfall traps. One trap (a 1 dl plastic jar with inner diameter of 56 mm and a depth of 70 mm) was set in a representative location within each 100 m2 tree-sampling plot, which yielded 6 traps per treatment per mire, i.e. 162 traps in total. Trapping was continuous for six weeks, with catches collected and traps reset every second week, between May and July 2007. To remove the surface tension and preserve the arthropods caught, 5 cl of a 10% NaCl solution with a few drops of detergent was added to the traps. Ants were identified with the keys of Collingwood (1979), Seifert (2000; 2007) and Czechowski et al. (2002). On the basis of published data (Krogerus 1960; Vepsäläinen et al. 2000; Punttila and Kilpeläinen 2009) and our own field experience, all the frequently collected ant species were ranked according to their affinity for mire habitats, response to drainage and affinity for pine heath forests on a scale from 1 (strongest mire affinity) to 6 (strongest pine-forest affinity). Additionally, when analysing the effects of drainage and restoration on the number of mire-ant species, we included only those species assessed in the latest red-list work of Finland by the expert group for Hymenoptera (Rassi et al. 2010) to live primarily in mires: Formica exsecta Nylander, 1846, F. picea Nylander, 1846, F. uralensis Ruzsky, 1895 and Myrmica scabrinodis Nylander, 1846 in our data.
2.3 Statistical analyses
First, we used log-likelihood ratio tests and Kruskal-Wallis non-parametric median tests to explore variation in the tree-stand variables and other variables characterizing mire habitats, and in ant occurrence among the treatments. To control for false discovery rate in multiple testing, the original p-values were adjusted with the method in Benjamini and Yekutieli (2001) using the function p.adjust (method = “BY”) in the package stats in R version 3.0.1 (R Core Team 2013). Additionally, we analysed ant occurrence among different mire types with Kruskal-Wallis non-parametric median tests to increase our limited knowledge of habitat associations of mire ants. We excluded all sampling locations on restored mires and all mire types with less than four sampling locations, which resulted in 89 sampling locations in these analyses.
Second, we applied non-metric multidimensional scaling (NMDS) to characterize differences in tree stands, floristic composition and ant-assemblage composition among the treatments (pristine, drained and restored). The three NMDS ordinations were performed using the vegan community ecology package, version 2.0–8 (Oksanen et al. 2013) in R (R Core Team 2013). We used the vegan function metaMDS with monoMDS to produce two-dimensional NMDS ordinations with several hundred random starts to find stable solutions. The ordination dimensions (“axes”) were scaled to half-change units. We fitted a set of environmental variables (see above) into ordinations using the vegan function envfit and tested their fit with permutation tests.
For the NMDS of the tree-stand characteristics, the Gower dissimilarity measure was used; the data were the number of sapling and tree individuals per species, the number of sapling and tree species, the number of trees in three diameter and three height classes, the number of snags in two diameter classes (< 7 cm, 7–20 cm), and the number of logs in three diameter classes in the 100 m2 tree-sampling plots and 25 m2 sapling squares. We excluded variables with less than 8 positive values out of 162 data entries.
For the NMDS of the floristic data, the Bray-Curtis dissimilarity measure, square root transformation and Wisconsin double standardization were used.
For the NMDS of the ant data, the Raup dissimilarity measure with ant presence-absence data in the pitfall traps was used, because conspecific individuals of social insects captured in a pitfall trap are not statistically independent units and do not directly reflect assemblage structure (Melbourne 1999; Vepsäläinen et al. 2000; Gotelli et al. 2011; Higgins and Lindgren 2012). Only worker-ant data were included in the NMDS and other multivariate analyses because only workers indicate established colonies.
Third, we used generalized linear mixed models (GLMM, glmer function in the lme4 package, Bates et al. 2013) in R (R Core Team 2013) to evaluate the effects of treatment and a set of environmental variables on the occurrence of ant species. Ant presence-absence data were modeled following a binomial error distribution. Mires nested within study regions were included as a random factor. Nine species occurring in > 10% of the traps were analysed individually.
GLMM analyses were subject to model selection, but treatment, Sphagnum cover, hummock cover and number of tall trees (> 3 m) not, owing to their a-priori expected importance for the individual species analysed. The following predictor variables were, however, subject to model selection: number of dead trees (logs and snags pooled), litter cover, covers of low (< 20 cm) and tall (> 20 cm) dwarf shrubs and the pooled cover of herbs, sedges and grasses. These variables were removed, one at a time, if their p-values exceeded 0.1 and if AIC values (see Burnham and Anderson 2002) decreased after excluding the particular variable. All predictor variables (except treatment) were standardized to zero mean and unit variance (Schielzeth 2010) to make them comparable. Mires from which no individuals of a particular species were caught were not included in the GLMM analyses since they may be unsuitable for that species for reasons that are not considered in this study.
3 Results
3.1 Mire-site types
Prior to starting restoration in 2003, 21.6% of the 162 sample locations represented transformed drained mires where the identification of the original mire type was impossible owing to successional changes. For the 34.0% and 43.8% of the sample locations established in pristine or transforming mire types, respectively (Table 1), pine mires and pine bogs were dominating (57.4% of all 162 locations), and pine fens and rich pine fens made up 18.5%. The most common pristine types were Sphagnum fuscum bogs (RaR, 13.0% of all sample locations, Table 1) and low-sedge pine fens (LkR, 9.9%), and the most common transforming types were transforming dwarf shrub pine bogs (muIR, 25.9%) and transforming Sphagnum fuscum bogs (muRaR, 10.5%).
3.2 The effects of drainage and restoration on tree stand and vegetation
Drainage led to a four-fold increase in the number of tree stems relative to pristine mires, and the increase in birch reduced the dominance of pines and led to mixed pine-birch stands in the drained mires (Table 2). Tree growth and thus growing stock increased, as reflected especially by the greater abundance of larger trees (d1.3 > 7 cm or h > 3 m) in drained than in pristine mires. Restoration harvesting successfully converted stand structure (in terms of stem number, tree-size distribution and tree-species composition) closer to pristine conditions (Table 2). Similarly, there were more birch saplings in the drained than pristine mires, whereas restored mires had intermediate numbers (Table 2). The amount of dead trees was generally low and rather similar among the treatments, but the amount of small-sized logs was higher in drained and restored than pristine mires (Table 2).
| Table 2. Mean ( | ||||||||||||
| Variable | Treatment | Test statistics | ||||||||||
| Pristine (N = 54) | Drained (N = 48) | Restored (N = 60) | ||||||||||
| SE | SE | SE | H | p adj. | ||||||||
| Living trees (h > 1.5 m): | ||||||||||||
| Total number of stems | 5.8 | 1.0 | a | 23.1 | 2.5 | b | 9.2 | 1.2 | a | 60.47 | <0.0001 | |
| No. of pines | 5.8 | 1.0 | a | 14.9 | 1.3 | b | 8.0 | 1.2 | a | 42.03 | <0.0001 | |
| No. of birches | 0.1 | 0.0 | a | 7.4 | 2.3 | b | 1.2 | 0.4 | a | 42.95 | <0.0001 | |
| No. of stems d1.3 < 7 cm | 4.7 | 0.8 | a | 13.8 | 2.3 | b | 7.1 | 1.1 | a | 17.65 | 0.0007 | |
| No. of stems d1.3 7–20 cm | 1.0 | 0.3 | a | 8.2 | 0.8 | b | 1.8 | 0.3 | a | 67.34 | <0.0001 | |
| No. of stems d1.3 > 20 cm | 0.0 | 0.0 | a | 1.1 | 0.2 | b | 0.3 | 0.1 | a | 32.44 | <0.0001 | |
| No. of stems h 1.5–3 m | 4.1 | 0.7 | 3.5 | 0.8 | 3.5 | 0.8 | 2.05 | 1.0000 | ||||
| No. of stems h 3–8 m | 1.7 | 0.3 | a | 12.5 | 2.0 | b | 4.9 | 0.7 | c | 40.24 | <0.0001 | |
| No. of stems h > 8 m | 0.0 | 0.0 | a | 7.0 | 1.2 | b | 0.8 | 0.4 | a | 68.29 | <0.0001 | |
| Number of species | 0.9 | 0.1 | a | 1.8 | 0.1 | b | 1.0 | 0.1 | a | 53.10 | <0.0001 | |
| Dead trees: | ||||||||||||
| Total number of snags | 0.7 | 0.2 | 1.1 | 0.2 | 0.9 | 0.3 | 1.49 | 1.0000 | ||||
| No. of snags d1.3 < 7 cm | 0.6 | 0.1 | 0.9 | 0.2 | 0.8 | 0.3 | 0.96 | 1.0000 | ||||
| No. of snags d1.3 7–20 cm | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | 1.16 | 1.0000 | ||||
| Total number of logs | 0.0 | 0.0 | a | 0.4 | 0.2 | a | 0.6 | 0.2 | a | 10.24 | 0.0354 | |
| No. of logs d1.3 < 7 cm | 0.0 | 0.0 | a | 0.4 | 0.2 | a | 0.6 | 0.2 | a | 11.15 | 0.0235 | |
| No. of logs d1.3 7–20 cm | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 1.57 | 1.0000 | ||||
| No. of logs d1.3 > 20 cm | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.70 | 1.0000 | ||||
| Tree saplings (h 50–150 cm): | ||||||||||||
| Total number of saplings | 1.9 | 0.3 | 2.6 | 0.6 | 1.7 | 0.3 | 1.02 | 1.0000 | ||||
| No. of pines | 1.9 | 0.3 | a | 1.4 | 0.4 | b | 1.2 | 0.2 | ab | 8.60 | 0.0736 | |
| No. of birches | 0.0 | 0.0 | a | 0.9 | 0.4 | b | 0.3 | 0.1 | ab | 17.14 | 0.0014 | |
| Number of species | 0.7 | 0.1 | 0.9 | 0.1 | 0.8 | 0.1 | 1.31 | 1.0000 | ||||
| Microsite types (%): | ||||||||||||
| Hummock | 46.1 | 3.6 | a | 96.6 | 2.0 | b | 66.9 | 4.1 | c | 71.47 | <0.0001 | |
| Lawn | 37.8 | 4.0 | a | 1.1 | 0.7 | b | 24.8 | 3.8 | c | 58.68 | <0.0001 | |
| Flark | 15.5 | 3.6 | a | 0.0 | 0.0 | b | 6.3 | 1.7 | a | 27.05 | <0.0001 | |
| Mire-surface coverage (%): | ||||||||||||
| Water | 0.0 | 0.0 | a | 0.0 | 0.0 | a | 2.4 | 1.0 | a | 14.20 | 0.0052 | |
| Litter | 2.4 | 1.2 | a | 12.1 | 3.1 | ab | 15.5 | 2.4 | b | 27.30 | <0.0001 | |
| Sphagnum spp. | 90.0 | 1.7 | a | 30.7 | 3.8 | b | 46.0 | 3.9 | b | 83.39 | <0.0001 | |
| Other mosses | 3.1 | 0.6 | a | 38.4 | 3.9 | b | 22.7 | 2.8 | c | 64.53 | <0.0001 | |
| Herbs, sedges and grasses | 14.0 | 1.1 | a | 8.2 | 1.0 | b | 20.9 | 2.2 | a | 22.51 | <0.0001 | |
| Low dwarf shrubs | 9.6 | 1.2 | ab | 16.6 | 2.1 | a | 8.6 | 1.3 | b | 8.92 | 0.0655 | |
| Tall dwarf shrubs | 3.7 | 0.6 | a | 6.2 | 0.8 | ab | 9.5 | 1.0 | b | 19.39 | 0.0007 | |
| Water-table depth (cm below the mire-surface) | 15.1 | 1.3 | a | 38.0 | 1.5 | b | 16.0 | 1.3 | a | 74.34 | <0.0001 | |
The microsite-type distribution of drained and pristine mires differed considerably from one another: the surface of drained mires was almost completely (97%, Table 2) covered by hummock, whereas more than half of the surface of pristine mires was covered by lawn (38%) and flark (16%). The microsite-type distribution in restored mires resembled that of pristine mires, but the share of hummock was larger (67%) than in pristine mires (46%).
Similarly, the surface cover of drained mires was dramatically different from pristine mires: the moss layer was dominated by Sphagnum mosses (90%, Table 2) in pristine mires, in drained mires 31%, with a higher cover of other mosses (38%). Differences in the herb layer were clear: the pooled cover of herbs, sedges and grasses was lower and the cover of tall dwarf shrubs higher in drained than pristine mires (Table 2). Pristine mires had higher cover of Sphagnum mosses but lower cover of other mosses, litter and tall dwarf shrubs than restored mires (Table 2).
The water-table level was lower in the drained (at a depth of 38 cm, Table 2) than in the pristine (15 cm) and restored (16 cm) mires.
Tree-stand and sapling characteristics of the drained mires differed clearly from those of the pristine and restored mires in the NMDS ordination (treatment, r2 = 0.205, p < 0.001): sampling locations of the drained mires were to the right in the ordination and displayed large scatter, whereas locations of the pristine and restored mires were to the left with small scatter (Fig. 1 A). Drained mires were characterized by a birch mixture and an abundance of large trees, but pristine and restored mires by pines and smaller trees (Fig. 1 B). The scatter of the restored sampling locations, however, was larger than that of the pristine ones (Fig. 1 A). Five of the 15 a-priori selected and fitted environmental variables showed significant correlations (p < 0.001) with the NMDS-ordination space: the number of tall trees, water-table depth and the cover of hummock microsite type had higher values in the drained mires than elsewhere (Fig. 1 A). The values of pooled cover of Sphagnum mosses and those of herbs, sedges and grasses were higher in the pristine and restored mires than in the drained ones (Fig. 1 A).
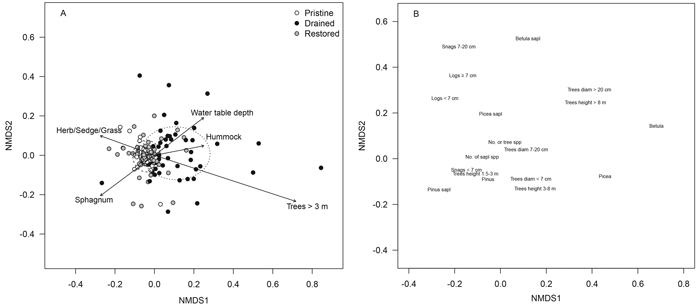
Fig. 1. NMDS ordination plots of the tree-stand variables presenting (A) sampling locations of the pristine (white dots), drained (black dots) and restored (grey dots) mires and (B) tree and sapling variables within the sampling locations. The dispersion ellipses in plot A indicate 1 SD of the weighted average of the site scores of pristine (solid line), drained (dotted line) and restored (dashed line) mires. The arrows in plot A indicate the environmental variables fitted to the ordination space such that only variables with highly significant p-values are shown (p < 0.001; the direction of the arrow indicates the direction of the gradient, and the length of the arrow indicates the strength of the correlation). View larger in new window/tab.
The floristic composition of the drained mires differed clearly from both the pristine and restored mires in the NMDS ordination (treatment, r2 = 0.352, p < 0.001, Fig. 2 A). Sampling locations of the drained mires were mostly to the right in the ordination, whereas those of the pristine mires were mostly to the left, and those of the restored mires mostly in between (Fig. 2 A). Sampling locations to the right, the drained mires and many of the restored ones had higher cover and occurrence rate of forest species such as Vaccinium myrtillus, V. vitis-idaea, Pleurozium schreberi (Willd. ex Brid.) Mitt. and Dicranum polysetum Sw. ex anon., whereas species characterizing pristine mires such as Carex limosa L., C. pauciflora Lightf., Drosera rotundifolia L., Sphagnum balticum (Russow) C.E.O.Jensen, S. fallax (H.Klinggr.) H.Klinggr., S. fuscum (Schimp.) H.Klinggr., S. papillosum Lindb., S. rubellum Wilson and V. microcarpum were to the left in ordination (Fig. 2 B, Appendix 1). Restoration seemed to have increased the covers and occurrence rates of mire species such as Sphagnum russowii Warnst., S. fallax and Polytrichum commune Hedw. in the short term as seen by their locations in the top half of the ordination (Fig. 2 B, Appendix 1).
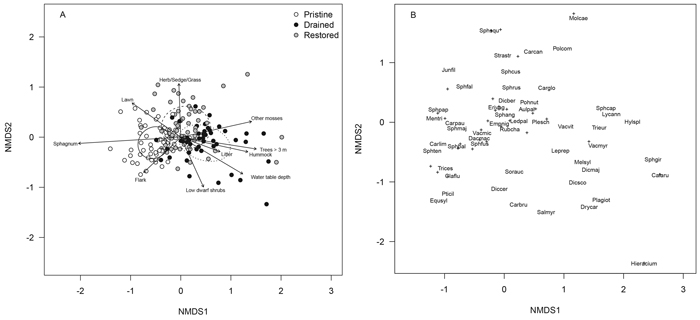
Fig. 2. NMDS ordination plots of the floristic data presenting (A) sampling locations of the pristine (white dots), drained (black dots) and restored (grey dots) mires and (B) moss, lichen and vascular plant species within the sampling locations (note the differences in the scales of axes between plots A and B). The dispersion ellipses in plot A indicate 1 SD of the weighted average of the site scores of pristine (solid line), drained (dotted line) and restored (dashed line) mires. The arrows in plot A indicate the environmental variables fitted to the ordination space such that only variables with highly significant p-values are shown (p < 0.001). The species names indicated in plot B are such that for overlapping labels, priority is given to the most abundant species and the rest are indicated with “+”. After this, 14 frequent species (occurring in > 9% of sampling locations) remained without labels. These species are located as follows: Dicranum polysetum ca. 0.7 units right from the origin, Vaccinium uliginosum, Chamaedaphne calyculata, Polytrichum strictum Menzies ex Brid., Vaccinium oxycoccos, Betula nana, Sphagnum magellanicum Brid. and Andromeda polifolia within ca. 0.3 units from the origin, Carex rostrata Stokes ca. 1.0 units left from the origin, Pinus sylvestris L., Calluna vulgaris, Mylia anomala (Hook.) Gray, Drosera rotundifolia and Sphagnum rubellum within ca. 0.5–1.0 units toward the ca. lower left corner from the origin. For species abbreviations, see Appendix 1 (abbreviations represent the first three letters of the genus name and the first three letters of the species name). View larger in new window/tab.
A number of species located in the top half of the ordination have benefitted from recent disturbances during restoration, e.g. Carex globularis L., Polytrichum commune, Sphagnum squarrosum Crome and Straminergon stramineum (Dicks. ex Brid.) Hedenäs (Fig. 2 B, Appendix 1). Additionally, the cover of especially Eriophorum vaginatum L. seemed to have increased considerably in restored mires relative to both drained and pristine mires although there were no great differences in its occurrence rate among the treatments (Appendix 1).
The same five a-priori selected environmental variables as in the tree-stand NMDS showed significant correlations (p < 0.001) here and correlated similarly with the vegetation ordination space as above, but also five additional variables correlated significantly with the ordination (Fig. 2 A): low dwarf shrubs and litter tended to increase in cover towards the drained mires, other mosses than Sphagnum spp. tended to increase in cover towards the drained and restored mires, and the cover of the microsite type flark increased towards the pristine mires and that of lawn towards both pristine and restored mires. Generally, the ordination revealed a gradient from more wet and fertile conditions characterized by a high cover of lawn and flark microsites to drier and poorer conditions with a high cover of hummock microsites (Fig. 2 A).
3.3 Ant occurrence among mire-site types
The ant data comprised 20 species: 10 579 workers of 17 species, 169 queens of 13 species and two males of one species (Appendix 2). The most abundant species in the worker data were Lasius platythorax Seifert, 1991, Formica uralensis, Myrmica ruginodis Nylander, 1846, M. scabrinodis and F. sanguinea Latreille, 1798, and the most frequently caught species were M. ruginodis (66% of the 162 sampling locations), M. scabrinodis (65%), L. platythorax (49%), Camponotus herculeanus (Linnaeus, 1758) (33%), and Leptothorax acervorum (Fabricius, 1793) (22%).
The species ranked a-priori to have the strongest pine-forest affinities, C. herculeanus and M. ruginodis, were more frequent in transforming and transformed than in pristine mire types (worker data, Table 3). Species ranked to have the strongest mire affinities showed more variable pattern: F. picea occurred almost exclusively in pristine mire types, whereas F. uralensis and M. scabrinodis were found frequently in both pristine and transforming and transformed mire types (Table 3). The rest of the species seemed to occur more evenly among the mire types (Table 3). Queens of the forest species M. ruginodis were more frequent in the transforming and transformed than in the pristine mire types, whereas queens of M. scabrinodis showed the opposite pattern (Table 3). The number of all ant species did not differ among mire types, whereas the number of mire ant species was higher in pristine mire types than in transforming and transformed types, and seemed to decrease with increasing growing stock (Table 3).
| Table 3. Mean ( | ||||||||||||||||||||||||||
| Species | Mire type1 | Test statistics | ||||||||||||||||||||||||
| Pristine | Transforming and transformed | |||||||||||||||||||||||||
| LkR (N = 15) | RaR (N = 21) | SR (N = 4) | TR (N = 6) | muRaR (N = 7) | muIR (N = 16) | TKg (N = 20) | ||||||||||||||||||||
| fr | fr | fr | fr | fr | fr | fr | G2 | p adj. | ||||||||||||||||||
| Ant workers | ||||||||||||||||||||||||||
| Formica picea | 0.5 | 7 | 0.3 | 7 | 0.8 | 3 | 0.3 | 2 | 0.0 | 0 | 0.0 | 0 | 0.1 | 1 | 27.30 | 0.0010 | ||||||||||
| Formica uralensis | 0.1 | 2 | 0.0 | 0 | 0.0 | 0 | 0.2 | 1 | 0.3 | 2 | 0.2 | 3 | 0.2 | 3 | 8.66 | 0.7269 | ||||||||||
| Myrmica scabrinodis | 0.6 | 9 | 0.9 | 18 | 0.8 | 3 | 0.7 | 4 | 0.9 | 6 | 0.6 | 10 | 0.4 | 7 | 13.90 | 0.1592 | ||||||||||
| Lasius platythorax | 0.3 | 4 | 0.4 | 9 | 0.5 | 2 | 0.5 | 3 | 0.3 | 2 | 0.5 | 8 | 0.5 | 10 | 3.25 | 1.0000 | ||||||||||
| Formica sanguinea | 0.1 | 2 | 0.4 | 9 | 0.3 | 1 | 0.0 | 0 | 0.4 | 3 | 0.1 | 1 | 0.1 | 1 | 16.86 | 0.0579 | ||||||||||
| Leptothorax acervorum | 0.2 | 3 | 0.4 | 9 | 0.5 | 2 | 0.2 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | 2 | 11.43 | 0.3491 | ||||||||||
| Myrmica rubra | 0.1 | 2 | 0.1 | 2 | 0.3 | 1 | 0.0 | 0 | 0.0 | 0 | 0.1 | 1 | 0.2 | 3 | 4.43 | 1.0000 | ||||||||||
| Myrmica ruginodis | 0.3 | 5 | 0.4 | 9 | 0.0 | 0 | 0.3 | 2 | 0.9 | 6 | 0.9 | 15 | 0.9 | 18 | 36.74 | < 0.0001 | ||||||||||
| Camponotus herculeanus | 0.1 | 1 | 0.0 | 0 | 0.0 | 0 | 0.2 | 1 | 0.1 | 1 | 0.8 | 12 | 0.7 | 14 | 51.34 | < 0.0001 | ||||||||||
| Ant queens | ||||||||||||||||||||||||||
| Myrmica scabrinodis | 0.2 | 3 | 0.3 | 7 | 1.0 | 4 | 0.5 | 3 | 0.1 | 1 | 0.3 | 4 | 0.0 | 0 | 25.74 | 0.0017 | ||||||||||
| Myrmica ruginodis | 0.1 | 2 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.3 | 2 | 0.6 | 9 | 0.5 | 9 | 29.93 | < 0.0001 | ||||||||||
| Mean number of species | H | p adj. | ||||||||||||||||||||||||
| Mire ants | 1.3 | 0.2 | 1.2 | 0.1 | 1.8 | 0.3 | 1.3 | 0.4 | 1.1 | 0.1 | 0.9 | 0.2 | 0.6 | 0.1 | 20.45 | 0.0158 | ||||||||||
| All ants | 2.4 | 0.3 | 3.0 | 0.3 | 3.3 | 0.5 | 2.5 | 0.8 | 3.3 | 0.4 | 3.5 | 0.3 | 3.6 | 0.3 | 8.82 | 0.7269 | ||||||||||
| 1Mire type abbreviations are according to Eurola et al. (1995), and English translations are according to Raunio et al. (2008): LkR = Low-sedge pine fens, RaR = Sphagnum fuscum bogs, SR = Tall-sedge pine fens, TR = Eriophorum vaginatum pine bogs, muRaR = Transforming Sphagnum fuscum bogs, muIR = Transforming Dwarf shrub pine bogs, TKg = Transformed drained mires. | ||||||||||||||||||||||||||
3.4 The effects of drainage and restoration on ants
The mean number of mire-ant species was highest in pristine mires, but the number of all ant species was highest in drained mires (Table 4). The mire species Formica picea occurred almost exclusively in the pristine mires, and the queens of another mire species, Myrmica scabrinodis, were most frequent in pristine mires, but this pattern was not observed for its workers (Table 4). The workers of the forest species Camponotus herculeanus and M. ruginodis – and queens of the latter – were most frequent in drained mires and common also in restored mires. Occurrence rate peaked in restored mires only for C. herculeanus queens (Table 4). The occurrence rates of the rest of the species did not differ statistically significantly among the treatments (Table 4).
| Table 4. Mean ( | |||||||||||
| Species | Treatment | Test statistics | |||||||||
| Pristine (N = 54) | Drained (N = 48) | Restored (N = 60) | |||||||||
| fr | fr | fr | G2 | p adj. | |||||||
| Ant workers: | |||||||||||
| Formica picea | 0.44 | 24 | 0.02 | 1 | 0.02 | 1 | 48.63 | < 0.0001 | |||
| Formica uralensis | 0.07 | 4 | 0.19 | 9 | 0.08 | 5 | 3.76 | 0.6328 | |||
| Myrmica scabrinodis | 0.74 | 40 | 0.52 | 25 | 0.67 | 40 | 5.49 | 0.2918 | |||
| Lasius platythorax | 0.35 | 19 | 0.50 | 24 | 0.60 | 36 | 7.13 | 0.1431 | |||
| Formica sanguinea | 0.22 | 12 | 0.13 | 6 | 0.23 | 14 | 2.44 | 1.0000 | |||
| Leptothorax acervorum | 0.30 | 16 | 0.15 | 7 | 0.20 | 12 | 3.52 | 0.6513 | |||
| Myrmica rubra | 0.09 | 5 | 0.13 | 6 | 0.10 | 6 | 0.30 | 1.0000 | |||
| Myrmica ruginodis | 0.37 | 20 | 0.92 | 44 | 0.72 | 43 | 37.34 | < 0.0001 | |||
| Camponotus herculeanus | 0.06 | 3 | 0.67 | 32 | 0.30 | 18 | 47.23 | < 0.0001 | |||
| Ant queens: | |||||||||||
| Myrmica scabrinodis | 0.41 | 22 | 0.10 | 5 | 0.18 | 11 | 14.25 | 0.0061 | |||
| Myrmica ruginodis | 0.04 | 2 | 0.42 | 20 | 0.28 | 17 | 24.99 | < 0.0001 | |||
| Camponotus herculeanus | 0.11 | 6 | 0.02 | 1 | 0.20 | 12 | 9.68 | 0.0450 | |||
| Mean number of species: | H | p adj. | |||||||||
| Mire ants | 1.3 | 0.1 | a | 0.8 | 0.1 | b | 0.8 | 0.1 | b | 22.59 | < 0.0001 |
| All ants | 2.7 | 0.2 | a | 3.6 | 0.2 | b | 3.1 | 0.1 | ab | 10.23 | 0.0390 |
Of the nine species analysed individually with GLMM, three were ranked a priori to have the strongest mire affinities (rank 1: F. picea, F. uralensis and M. scabrinodis), two were ranked a priori to have the strongest pine-forest affinities (rank 6: M. ruginodis and C. herculeanus), and the remaining four species to be more generalists in their habitat affinities (rank 2: L. platythorax, F. sanguinea and rank 3: L. acervorum, species that occur both in mires and forests but disappear with tree-canopy closure during forest succession, and rank 4: M. rubra (Linnaeus, 1758), which is known to tolerate tree-canopy shading). Below, we focus on the overall trends of occurrence of species along the mire-forest continuum, rather than statistically significant differences in the occurrence rate of individual species.
Mire specialist species showed variable responses to treatment (Fig. 3 A, Table 5). Formica picea responded statistically significantly and negatively to restored sites, and the trend for F. uralensis suggests negative association with restored mires. Interestingly, M. scabrinodis and F. uralensis tended to associate with drained sites. The fairly generalist species, L. platythorax responded statistically significantly and positively to restored sites, and the trend for F. sanguinea suggests that it associates with restored sites (Fig. 3 B, Table 5). Myrmica rubra behaved like the forest-associated species, whereas L. acervorum responded more as a mire specialist (Fig. 3 B).
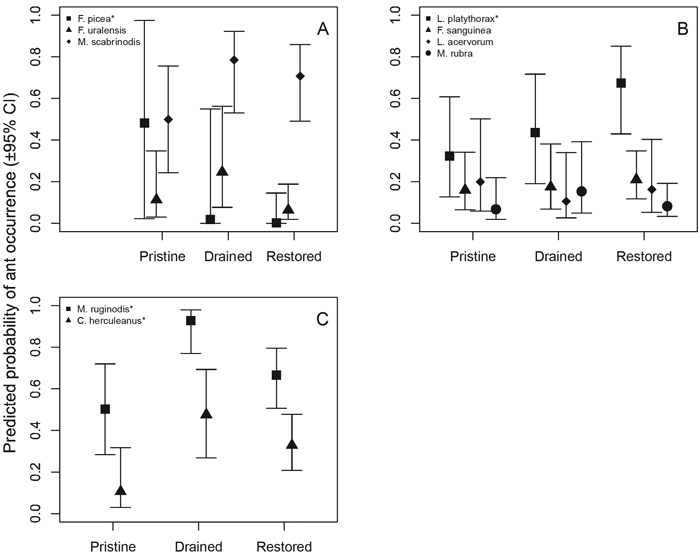
Fig. 3. Statistical responses of individual ant species to mire treatment (A = mire specialist species, B = generalist species, C = forest species). * = statistically significant (p < 0.05) responses (see Table 5).
| Table 5. Generalised linear mixed model results of ant mire specialists, generalists and forest species. Model estimates, standard errors (SE) and p-values of terms retained in the models are given. Treatment, Sphagnum cover, hummock cover and number of trees (> 3 m) were a priori chosen as important environmental variables for the ant occurrences and were retained in all final models. Other variables were subject to model selection and their values are only given when they remained in the final model. Statistically significant p-values (< 0.05) are in bold. The intercept represents prediction in the pristine mire treatment. Est. = Estimate. * = mire specialist species, ** = generalist species, *** = forest species, all according to our a priori evaluations. View in new window/tab. |
As expected, the two forest-associated species, C. herculeanus and M. ruginodis, occurred statistically significantly more often in drained sites, less in restored sites and least frequently in pristine sites (Fig. 3 C).
Ants responded statistically significantly to a number of variables tested, although the species seemed to differ in sensitivity (Table 5). Only the mire specialist M. scabrinodis responded significantly and positively to Sphagnum moss cover, although there is a suggestive trend of mire specialist species being positively associated with Sphagnum cover and forest species the opposite way (Fig. 4 A). The number of tall trees (> 3 m) seemed to affect the majority of species as expected a priori: the probability of occurrence of mire specialist species tended to decrease and that of forest species to increase with an increase in the number of tall trees (Fig. 4 B). Two species responded statistically significantly to the number of tall trees: as predicted, the mire specialist M. scabrinodis negatively and the forest species C. herculeanus positively. None of the species responded statistically significantly to hummock cover, although the mire specialist F. picea and the generalist L. platythorax appeared to respond positively and the forest species M. ruginodis negatively to hummock cover (Fig. 4 C, Table 5). Additionally, a few other variables remained in the final models, but for only two species the responses were statistically significant: C. herculeanus showed a negative response to dead trees, and M. ruginodis responded positively to low and tall dwarf shrubs and annual plants (Table 5).
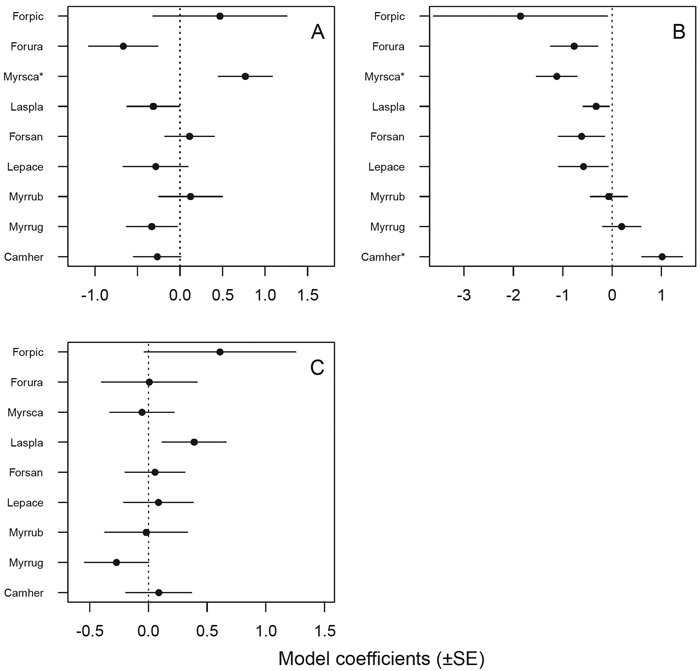
Fig. 4. Statistical responses (model coefficients ± SE, see Table 5) of individual ant species to environmental variables: A = Sphagnum moss cover, B = number of tall trees (> 3 m), C = hummock cover. * = statistically significant (p < 0.05) responses. Species were listed a priori (based on expert opinion and the literature: Krogerus 1960; Vepsäläinen et al. 2000; Punttila and Kilpeläinen 2009) from the most mire-associated (mire specialist) species at the top of each plot to species with the strongest forest affinities (forest species) at the bottom of each plot; generalist species are located in-between.
The ant-assemblage structure of the drained mires differed from the pristine mires in the NMDS ordination (treatment, r2 = 0.221, p < 0.001), but the scatter of the sampling locations was large in each of the three treatments, and the locations of the restored mires overlapped with those of both pristine and drained ones (Fig. 5 A). There seems to be a naturalness gradient from the sampling locations of pristine mires (to the right) towards those of drained mires (to the left), with the locations of restored mires in-between (Fig. 5 A). Seven of the 15 fitted environmental variables correlated significantly (p < 0.001) with the NMDS-ordination space: the cover of Sphagnum mosses and that of the microsite type flark increased towards the pristine locations, whereas the rest of the statistically significant variables were related to drainage and correlated approximately in the opposite direction, towards the drained and restored mires (Fig. 5 A). Ant assemblages differed from each other such that the more forested mires seemed to be located to the left-hand side (correlation with tall trees) and the more open mires with abundant lower trees more to the top of the ordination.
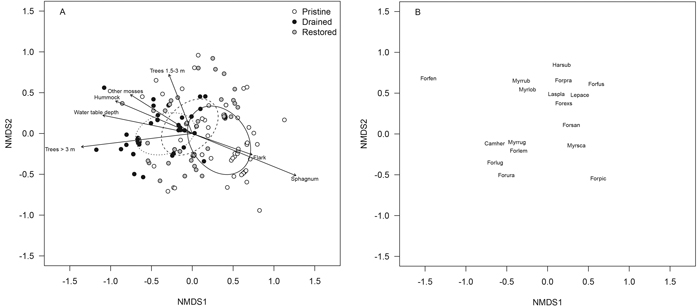
Fig. 5. NMDS ordination plots of the ant data presenting (A) sampling locations of the pristine (white dots), drained (black dots) and restored (grey dots) mires and (B) ant species within the sampling locations. The dispersion ellipses in plot A indicate 1 SD of the weighted average of the site scores of pristine (solid line), drained (dotted line) and restored (dashed line) mires. The arrows in plot A indicate the environmental variables fitted to the ordination space such that only variables with highly significant p-values are shown (p < 0.001). For species abbreviations, see Appendix 2 (abbreviations represent the first three letters of the genus name and the first three letters of the species name). View larger in new window/tab.
Differences in ant assemblages among the non-pristine mires were expressed such that the shade-tolerant forest species C. herculeanus and M. ruginodis occurred in the more heavily forested sampling locations at the bottom left of the ordination, whereas species of open-canopy forests and mires – such as L. platythorax, Leptothorax acervorum, Formica sanguinea and F. exsecta – characterized the less densely forested sampling locations to the top right (Fig. 5 B). Open pristine mires were characterized by the occurrence of the mire species F. picea and M. scabrinodis, at the bottom right of the ordination (Fig. 5 B). Another mire species, F. uralensis, however, had benefitted from drainage at least temporarily, and is located closer to the forest species C. herculeanus and M. ruginodis in ordination (Fig. 5 B). Indeed, half of the occurrences of F. uralensis were from drained mires (Table 4).
4 Discussion
In general, our results showed predictable responses in both habitat structure – including vegetation – and ant assemblages to mire drainage and restoration. This conclusion is similar to the results in a mire restoration study dealing with a number of solitary invertebrate groups (Noreika et al. 2015). Taken together, our results are consistent with the Field of Dreams hypothesis (Palmer et al. 1997), which suggests that if you rebuild the habitat structure, the flora and fauna will return; here mire restoration resulted in rapid recovery of mire vegetation and ant assemblages (see also Noreika et al. 2015). However, for mire-specialist ant species the results were variable and longer-term monitoring is needed to evaluate the success of restoration. Considerable time lag – relative to solitary insects with shorter generation times – is expected in the response of ants, which form long-lived perennial colonies with long colony cycles.
4.1 Effects of drainage and restoration on mire-habitat structure
Restoration measures – the filling of ditches, heavy thinning and partial clear-cutting of tree stands – were successful in that both the water-table level and stand structure of restored mires matched those of pristine mires, although heavier cuttings of larger trees would have been required to accord better with pristine mires in terms of tree-canopy shading. The amount of dead wood was generally low in all mires, and the amount of small logs was only slightly higher in drained and restored mires than in pristine ones indicating that tree mortality due to competition had not yet started in drained mires, and that coarser logging residues (felled trees) were not left behind in restored mires in large quantities.
We showed that tree stand structure was more variable in the drained than in the pristine or restored areas (Fig. 1 A), which followed probably both from drainage and forest management activities. In the long run, forest management tends to homogenize stand structure by, e.g. decreasing variation in stem size distribution, whereas forest stands in pristine mires exhibit multi-modal structure (Uuttera et al. 1997).
The pristine microsite-type distribution was re-established a few years after restoration: the mire surface was almost completely covered by the hummock microsite type in drained mires, but was reduced to two thirds in restored mires, whereas it was 46% in pristine mires. Although habitat structure of the restored mires had clearly changed towards pristine conditions in a few years after restoration, the cover of Sphagnum mosses was still lower (similarly as 10 years after restoration in Haapalehto et al. 2011), and the cover of e.g. tall dwarf shrubs was still higher in restored than pristine mires (see Fig. 3 in Laine et al. 1995a).
Drainage seemed to have led to similar successional changes of mire vegetation as in earlier studies – increased occurrence and cover of forest species and the disappearance or decline of mire species (Laine et al. 1995a; b; Jauhiainen et al. 2002; Haapalehto et al. 2011; Haapalehto 2014). Similarly, a rapid change towards pristine floristic composition in restored mires has been observed in earlier studies. The strength of the response depends on the severity of degradation of the drained mires, and on mire fertility. Rapid responses have been restricted to less heavily degraded sites within restored mires, whereas no or only minor changes have been reported from heavily degraded sites (Komulainen et al. 1999; Jauhiainen et al. 2002; Haapalehto et al. 2011; Haapalehto 2014). In our data, the rapid recovery may have been enhanced by a lack of dispersal barriers, as the restored mires were parts of larger mires with varying proportions of unditched areas. These likely provide refugia for mire species in close proximity of the drained and restored mires, and thus they also serve as potential areas for recolonization of restored mires.
Similarly to earlier studies, some species, e.g. Eriophorum vaginatum, responded strongly by increasing in cover (Komulainen et al. 1999; Jauhiainen et al. 2002; Haapalehto et al. 2011). On the other hand, some bog species of wet hollows, e.g. Carex limosa and Scheuchzeria palustris L., seemed to be missing from restored mires in the short term (similarly as 10 years after restoration in Haapalehto et al. 2011), and in another study these species seemed not to have colonized restored mires even after half a century, despite the existence of nearby populations (Soro et al. 1999). Laine et al. (2011) monitored the effects of restoration on vegetation of oligotrophic pine fens in a more northern area in Kainuu, eastern Finland for two years. They showed that the vegetation of drained mires had not changed much from pristine conditions during 30 years of drainage: only the typical hollow species Sphagnum majus (Russow) C.E.O.Jensen, S. balticum and Scheuchzeria palustris were missing. Laine et al. (2011), however, also found that there were only minor differences in the floristic composition before vs. after restoration in the two-year monitoring period: the only statistically significant restoration-related change was that the cover of sedges increased in restored and decreased in pristine fens.
4.2 Ant occurrence in mire-site types
Knowledge on the ant fauna of mire types is limited (Oinonen 1956; Krogerus 1960). We showed the importance of tree cover on the occurrence of ant species among mire types: the occurrence rates of mire species tended to decrease with increasing growing stock, whereas forest species showed the opposite pattern (Table 3).
A NMDS analysis of the data of Krogerus (1960) revealed a clear gradient in the composition of ant assemblages from open fens and rich fens through pine mires and pine bogs to spruce mires (Punttila et al. 2013). Dlussky (2001) found a similar openness gradient within an oligotrophic bog in the Novgorod district, Russia. The ant assemblages of the open central parts of the bog were characterized by true (“endemic”) mire species (F. picea, F. uralensis and F. forsslundi Lohmander, 1949) and some “forest species” (L. platythorax, M. rubra, M. scabrinodis and L. acervorum), whereas pine forests of the mire edges were occupied by forest species (C. herculeanus, L. platythorax, M. rubra and F. polyctena Förster, 1850). This openness gradient resembles our NMDS-ordination results (Fig. 5 B), which showed that assemblages at the open end of the gradient were characterized by the mire species F. picea, M. scabrinodis and F. uralensis. On the other hand, the assemblages in sparsely forested areas with low trees were distinguished by species that colonize recently disturbed or naturally open forest areas (e.g. L. platythorax, L. acervorum, F. sanguinea, F. exsecta). Finally, the more heavily forested areas with tall trees were characterized by forest species that are capable of persisting through the whole cycle of forest succession, i.e. C. herculeanus and M. ruginodis (Niemelä et al. 1996).
4.3 Drainage and ants
The succession of tree stands on drained mires with increased growing stock of large trees and increased deciduous mixture of birch with pine result in shaded conditions. This change decimated colonies of mire-ant species requiring sun exposure, which prevails in pristine conditions of pine mires whereas shade-tolerant forest species, such as Camponotus herculeanus and Myrmica ruginodis, benefitted similarly as in earliers studies and thus, the ant assemblages of drained mires differed clearly from those of pristine mires (Krogerus 1960; Collingwood 1963; 1999; Vepsäläinen et al. 2000; Punttila and Kilpeläinen 2009). The mire specialist Formica picea in particular suffered from drainage and this is also expected in the long term for F. uralensis (which seems to benefit from the early stages of drainage) and M. scabrinodis (Krogerus 1960; Vepsäläinen et al. 2000). Krogerus (1960) found that F. picea inhabited the moistest parts of pine mires but F. uralensis the driest sites. Punttila and Kilpeläinen (2009) found that the occurrence rates of the three mound-building species restricted to mires in their data are highest in undrained pristine mires, lower in transforming mires and lowest in transformed ones (F. uralensis), or the species are missing from transformed mires (F. fennica Seifert, 2000 and F. forsslundi), whereas the most common species of forests on mineral soils, the wood ant F. aquilonia Yarrow, 1955, showed the opposite pattern. Similar results have been obtained in southern Sweden where the slow drying-out of bog areas has decimated the prevalence of the mire species F. uralensis, F. forsslundi and F. picea in only one decade (Collingwood 1999).
In our data, ant species of drier open habitats (e.g. open early successional forests after disturbances) and generalist ant species benefitted from the early phases of mire drainage. Most of these species disappeared with tree-canopy closure also during forest succession on mineral soils (Punttila et al. 1991), but in the early stages of drainage they seem to replace mire species. For instance, M. ruginodis replaced M. scabrinodis (Vepsäläinen et al. 2000) although M. scabrinodis appeared to be more resilient than other mire species (see also Collingwood 1999), and especially Lasius platythorax (Collingwood 1999; Dlussky 2001) but possibly also Formica fusca Linnaeus, 1758 and F. lemani Bondroit, 1917 seemed to replace F. picea, and in the later successional stages, F. aquilonia replaced territorial mound-building mire species (Vepsäläinen et al. 2000; Punttila and Kilpeläinen 2009). Open treeless mires or sparsely forested, drained open mires do not necessarily provide any habitat for wood ant species of the Formica rufa group (F. aquilonia, F. lugubris Zetterstedt, 1838, F. rufa Linnaeus, 1758, F. polyctena and F. pratensis Retzius, 1783) because arboreal aphid colonies providing nourishment for wood ants are scarce, and the foraging activity of wood ants may cease for long periods of time during high day-time temperatures in open mires and, finally, wood ants cannot find suitable below-ground overwintering sites deep enough because of the high water-table level (Vepsäläinen et al. 2000). In wooded and drained mires the situation is different which enables colonization of wood ants: when mire drainage proceeds, territorial wood-ant species of mineral soils also start to dominate transformed mires (Punttila and Kilpeläinen 2009). In our data, the wood ants F. lugubris and F. pratensis were missing from pristine mires, but these species occurred in seven (15%) and two (4%) sampling locations in drained, and in three (5%) and one (2%) locations in restored mires, respectively (Appendix 2).
4.4 Restoration and ants
We are not aware of any other study on the effects of mire restoration on ants, but with the results of the present study and the results of earlier studies concerning drained mires, together with information about the autecology, competitive capacity and dispersal of different ant species, we can outline some general principles to be taken into account when planning mire restoration.
The rewetting of mires and removal or strong thinning of the growing tree stock are key components in successful restoration of mire ants. A high water-table level prevents the overwintering of top-competitors of the territorial Formica ants of heath forests in mire habitats and thus leaves the terrain free for typical mire-ant assemblages to develop with their own top-competitors of other territorial Formica ants, such as F. uralensis, F. exsecta and F. forsslundi (see Punttila and Kilpeläinen 2009).
Another important issue in mire restoration for ants is the treatment of logging waste, i.e. tree stems, tops, branches and cut stumps after logging in naturally treeless or sparsely forested mires: whether these are left behind or harvested (Punttila et al. 2013).
Despite the fact that the amount of dead trees in our study was generally low in all treatments, the amount of small logs was somewhat higher in drained and restored mires than in pristine ones, thus providing more nesting sites for forest ants there. Most of the boreal forest ant species can nest in or even require dead wood for establishing new colonies, and for many species in many habitat types a large share of ant nests are located in dead wood (e.g. Oinonen and Wuorenrinne 1976; Franch and Espadaler 1988; Punttila and Haila 1996; Włodarczyk et al. 2009; Persson et al. 2013). Thus, leaving stumps, felled trees or logging residues in restored mires may enhance colonization of non-desired forest species in the restored mire habitats. Dead wood provides elevated microhabitats that may offer the only sun-exposed and warm above-ground nesting sites for forest ants. Such conditions are found in habitats where dense, shading herb-layer vegetation prevents sun exposure of the ground. In forested habitats, this may happen shortly after the creation of tree-canopy openings by wind throws, fire or cuttings, but this may also take place after disturbances – e.g. ditching or restoration – in mires.
Although the rapidly recovering moss layer of the restored mires may overgrow logging residues and cut stumps within a few years following restoration, they nevertheless provide luxuriant nesting substrates for a large variety of forest and generalist ant species. These newcomers may later prevent or slow down the colony establishment of mire specialist ant species by predation pressure on the dispersing mire-ant queens attempting colony founding, and through nest-site competition and pre-emption of nesting sites – such priority effects are exemplified, e.g. in the colonization by ants of small land uplift islands of the Baltic Sea (see Vepsäläinen and Pisarski 1982). These types of nesting substrates are missing almost entirely from pristine open mires and thus, their creation should be minimized in the restoration process. Ant species of open successional forests may also be capable of replacing the few remaining but weakened mire ant populations of restored mires. These forest species are also able to inhabit logging residues in the middle of larger restored mires, as the dispersing females of some of these species are rather strong fliers (Vepsäläinen and Pisarski 1982; Czechowski et al. 2013). The colonization of many aggressive and territorial Formica species is dependent on the occurrence of species in whose nests they start new colonies by temporary parasitism (Gösswald 1951; 1952; Kutter 1969; Wilson 1971; Collingwood 1979; Seifert 2000; 2007; Czechowski et al. 2012). These species can often utilize both mire specialist species remaining in the area, and generalist species of mineral soils, which have colonized the drained mire. Thus, their colonization may be enhanced in areas where both mire and forest species occur together (Collingwood 1979; Seifert 2007). Another option for logging-residue harvesting is to girdle the trees and let them die standing, which reduces the amount of potential dead-wood nesting sites for forest ant species by spreading the increase of logs on the ground over a longer time span.
In restoration planning, the surrounding terrestrial environment should also be taken into account to inhibit possible colonization of forest ants in restored mires. Restoration efforts can be timed to years when there are no larger regeneration cuttings or young sapling stands in close proximity to the restored areas. For instance, in southern Sweden, the eradication of Formica picea from slowly drying bogs was accelerated by colonization pressure and subsequent competitive exclusion by Lasius platythorax, which immigrated from the surrounding fresh thinning cuttings performed a few years earlier. In the cut area, the forest floor was covered by logging residues, fallen trees and sun-exposed cut stumps. Nearly all the cut stumps were occupied by flourishing L. platythorax colonies, and these ants were also seen in large quantities in bog tussocks in the mires (Collingwood 1999).
Our knowledge on the dispersal capacity and competitive ability of mire ants is scanty (but see Collingwood 1979; Vepsäläinen et al. 2000). Thus, it is difficult to predict the recolonization of restored mires by mire ants. Apparently dispersal among different habitat patches by winged females is rather limited at least in many polygynous ant species and populations (Sundström et al. 2005; Seppä 2008), but generally the dispersal capacity seems to vary widely from very strong dispersers (e.g. L. niger, C. herculeanus) to seemingly poor ones (see Vepsäläinen and Pisarski 1982). There is evidence that the dispersal of the mire ant Formica picea is weak, not only between different mires but also within mires (Mabelis and Chardon 2005; Rees et al. 2010). In our data, this species seemed to be almost exclusively confined to pristine mires. The recolonization of F. picea in restored mires probably depends heavily on the closeness of healthy populations. This may be critical for restoration because successful colonization of the rewetted mires by F. picea is a prerequisite for the subsequent colonization of the other true mire species, F. forsslundi and F. uralensis, which establish their new colonies through temporary parasitism in the nests of F. picea.
4.5 Habitat disturbance and ant colonization
Habitat disturbance often opens up new colonization possibilities for boreal ants in forested areas. Disturbances create favourable climatic conditions for species that require warm and well-lit conditions at least during the colonization stage. Large disturbances in mature forests also open competition-free terrain by often wiping out the territorial wood-ant colonies, whereas these colonies seem to tolerate smaller disturbances (Punttila et al. 1991; Punttila et al. 1994; Punttila 1996; Punttila et al. 1996; Sorvari and Hakkarainen 2005; 2007; Kilpeläinen et al. 2008; Punttila and Kilpeläinen 2009; Sorvari et al. 2011). In our data > 60% of Camponotus herculeanus queens were found in the samples of restored mires. Restored mires, however, should be inferior habitat for this wood-inhabiting species, because most of the trees have been removed from there, and the cut stumps are probably rather quickly buried under the growing mire mosses. Such colonization attempts may, however, be explained by possible attractiveness of recently disturbed open areas following restoration measures. Flying Camponotus queens might orientate towards light reflecting from disturbed habitat patches similarly as many other ant species do (Brian 1952; Pontin 1960; Brian et al. 1966; Wilson and Hunt 1966; Fowler 1987; Tschinkel 1987). In forested areas, recently disturbed areas devoid of territorial wood ants (Formica rufa group) are presumably optimal habitats for colony founding, and high queen numbers have indeed been observed in fresh clear-cuts following cutting and prescribed burning (Punttila et al. 1991; Punttila and Haila 1996). Also Lasius platythorax probably benefitted from recent disturbances in restored mires as suggested by our data.
Disturbed forested boreal areas are quickly colonized by many ant species requiring open conditions in at least the colony-founding stage, and thus the habitat becomes crowded such that the only options remaining for colony founding are nest parasitism and – for queens in established colonies – colony budding (Punttila et al. 1991). Such crowded conditions, however, seem not to be reached often in open pristine mires although these are long-lived habitats. Our own experience is that open pristine mires are often sparsely inhabited by ant colonies. The reason why apparently suitable nesting sites often remain uninhabited is not known, but presumably any larger ant-free terrain can be created by extreme events such as long-term flooding that drowns the ant colonies, or severe winters with thin snow cover lowering the overwintering survival of colonies, or wildfires in exceptionally dry years. The commonness of such events might ultimately determine the nest density of the mire. If this were the case, polygyny and subsequent budding, leading to multinest colonies, would increase the resilience of mire ant colonies in the long run, as the risk of extirpation of an extensive nest network should be smaller than that of a single nest (Mabelis and Chardon 2005).
On the other hand, ant assemblages in drained mires in our data had become more crowded than in pristine mires, as they housed populations of both forest and mire species. Thus, in both pristine and drained mires, nest budding (leading to multinest colonies) and nest parasitism are most likely as important modes of nest-founding as in successional boreal forests. In our data, the high frequency of Myrmica queens – M. scabrinodis in pristine and M. ruginodis in drained mires – indicated the occurrence of polygynous colonies, where the queens are moving or transferred among the nests of multinest colonies (Punttila et al. 1991; Seppä et al. 1995). Our data were, unfortunately, insufficient for making any observations on nest parasites because our sampling window was outside the dispersal period of most species, which is in the late season.
4.6 Conclusions
Generally, we tested whether restoring simple but crucial habitat characteristics, the water-table level by ditch filling and the naturally sparse and low pine stand by thinning and clear-cutting, would lead to recolonization and recovery of the characteristic vegetation and ant assemblages of pristine mire habitats. Here we cannot yet evaluate long-term restoration success, but our short-term results are consistent with predictions of the Field of Dreams hypothesis (Palmer et al. 1997), which does not necessarily apply to more heavily degraded mire ecosystems. The recovery of our study mires was fast because the drained mires still had an acrotelm (the surface layer of mire soil) and catotelm (core of peat), and mire organisms could recolonize the restored mires either from local refugia or from populations of nearby mires (see the discussion in Komulainen et al. 1999; Vasander et al. 2003). The connectedness of mire habitats, however, has been severely reduced especially in southern Finland; consequently, recolonization has become unlikely for many mire specialist species, which have become locally threatened (Rassi et al. 2010). Such demanding species in boreal areas may include the workerless social parasite Myrmica karavajevi (Arnoldi, 1930) and the temporary parasite M. vandeli Bondroit, 1920. Both species require dense colonies of their host ant Myrmica scabrinodis for which, especially within the boreal mainland areas of Finland, the most important habitat is mires (Vepsäläinen et al. 2000; Punttila et al. 2013). Degradation of mire habitats and their fragmentation may lead to the disappearance of these highly specialized rare social parasites and other mire specialist species from large areas. In Finland, the threat is most acute in the south, where almost 80% of mires have been drained and the distances between restored mires and pristine mires housing potential source populations are increasing with decreasing connectedness, which weakens the recolonization prospects of specialist species once they have disappeared (Vepsäläinen et al. 2000; Auvinen et al. 2007; Punttila et al. 2013).
Acknowledgements
We are grateful to Kaija Eisto, Tuomas Haapalehto, Reijo Hokkanen, Esko Hyvärinen, Reijo Kuosmanen, Jouni Penttinen, Jussi Päivinen, Teemu Rintala, Maarit Similä and Anneli Suikki from Metsähallitus Parks & Wildlife Finland (former Natural Heritage Services), Päivi Halinen of the Centre for Economic Development, Transport and the Environment of central Finland, and Atte Komonen of the University of Joensuu for participating in the planning of the study and for organizing many practicalities starting from study-area selection. We thank Liisa Karhu, Kari Lahtinen, Lauri Mikonranta and Tiina Virta for participation in the field work, and Simo Väänänen for identification of the ant samples and participation in the early stages of data processing and literature searches. We also thank Kaisu Aapala, Tuomas Lahti, Krister Karttunen, Tapani Sallantaus, Jari Teeriaho, Seppo Tuominen and Satu Turtiainen of the Finnish Environment Institute for indispensable scientific advice and practical help during the course of our study. Data collecting and ant identification were funded by Metsähallitus Parks & Wildlife Finland, and the studied mires belong to two LIFE Nature projects participated by Metsähallitus (Karelian mires and virgin forests – pearls in the chain of geohistory 2002–2007, and Protection of valuable bird-rich wetlands in Central Finland 2001–2006).
References
Aapala K., Sallantaus T., Haapalehto T. (2008). Ecological restoration of drained peatlands. In: Korhonen R., Korpela L., Sarkkola S. (eds.). Finland - Fenland: research and sustainable utilisation of mires and peat. Finnish Peatland Society, Helsinki. p. 243–249.
Aapala K., Lindholm T., Sallantaus T., Similä M., Tahvanainen T., Haapalehto T., Penttinen J., Salminen P., Suikki A., Pekka V. (2012). Monitoring restored peatlands in Finnish nature reserves. In: Lindholm T., Heikkilä R. (eds.). Mires from pole to pole. The Finnish Environment Insitute, Helsinki. The Finnish Environment 38. p. 197–204. https://helda.helsinki.fi/handle/10138/38728.
Ahti T., Hämet-Ahti L., Jalas J. (1968). Vegetation zones and their sections in northwestern Europe. Annales Botanici Fennici 5: 169–211.
Andersen A.N., Majer J.D. (2004). Ants show the way Down Under: invertebrates as bioindicators in land management. Frontiers in Ecology and the Environment 2: 291–298. http://dx.doi.org/10.1890/1540-9295(2004)002[0292:ASTWDU]2.0.CO;2.
Auvinen A.-P., Hildén M., Toivonen H., Primmer E., Niemelä J., Aapala K., Bäck S., Härmä P., Ikävalko J., Järvenpää E., Kaipiainen H., Korhonen K.T., Kumela H., Kärkkäinen L., Lankoski J., Laukkanen M., Mannerkoski I., Nuutinen T., Nöjd A., Punttila P., Salminen O., Söderman G., Törmä M., Virkkala R. (2007). Evaluation of the Finnish national biodiversity action plan 1997–2005. Monographs of the Boreal Environment Research 29. 54 p. https://helda.helsinki.fi/handle/10138/39337.
Auvinen A.-P., Kemppainen E., von Weissenberg M. (eds.) (2010). Fourth national report on the implementation of the convention on biological diversity in Finland. The Finnish Environment 3/2010. Ministry of the Environment. 191 p. https://helda.helsinki.fi/handle/10138/37978.
Bates D., Maechler M., Bolker B. (2013). lme4: linear mixed-effects models using S4 classes. http://CRAN.R-project.org/package=lme4.
Benjamini Y., Yekutieli D. (2001). The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29: 1165–1188.
Brian M.V. (1952). The structure of a dense natural ant population. Journal of Animal Ecology 21: 12–24. http://dx.doi.org/10.2307/1907.
Brian M.V., Hibble J., Kelly A.F. (1966). The dispersion of ant species in a southern English heath. Journal of Animal Ecology 35: 281–290. http://dx.doi.org/10.2307/2395.
Bujan J., Brigić A., Ješovnik A. (2010). Ant diversity in Croatian peat bogs. In: Nash D.R., den Boer S.P.A., De Fine Licht H.H., Boomsma J.J. (eds.). Abstracts for the XVI Congress of the International Union for the Study of Social Insects, 8–13 August, Copenhagen, Denmark. http://www.iussi.org/iussi2010/Media/IUSSI2010AbstractBook.pdf. p. 18.
Burnham K.P., Anderson D.R. (2002). Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York, NY, US.
CBD (2010). Decision X/2, the strategic plan for biodiversity 2011–2020 and the Aichi biodiversity targets. Convention on Biological Diversity, 18–29 October, Nagoya, Japan. http://www.cbd.int/decision/cop/?id=12268.
Collingwood C.A. (1963). Three ant species new to Norway. Entomologist’s Record and Journal of Variation 75: 225–228.
Collingwood C.A. (1979). The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica 8: 1–174.
Collingwood C.A. (1999). Changes in the ant (Hym.: Formicidae) fauna of a Swedish bogland area 1986–1997. Entomologist’s Record and Journal of Variation 111: 233–234.
Czechowski W., Radchenko A., Czechowska W. (2002). The ants (Hymenoptera, Formicidae) of Poland. Museum and Institute of Zoology PAS. (Polish Academy of Sciences), Warszawa. 200 p. + appendix.
Czechowski W., Radchenko A., Czechowska W., Vepsäläinen K. (2012). The ants of Poland with reference to the myrmecofauna of Europe. Fauna Poloniae 4. 496 p.
Czechowski W., Vepsäläinen K., Radchenko A. (2013). Ants on skerries: Lasius assemblages along primary succession. Insectes Sociaux 60: 147–153. http://dx.doi.org/10.1007/s00040-012-0278-y.
Dlussky G.M. (2001). Structure of ant community (Hymenoptera, Formicidae) from an oligotrophic peat bog. Zoologicheskii Zhurnal 80: 976–985. [In Russian with an English summary].
Dunn R.R. (2005). Modern insect extinctions, the neglected majority. Conservation Biology 19: 1030–1036. http://dx.doi.org/10.1111/j.1523-1739.2005.00078.x.
Ellison A.M., Farnsworth E.J., Gotelli N.J. (2002). Ant diversity in pitcher-plant bogs of Massachusetts. Northeastern Naturalist 9: 267–284. http://dx.doi.org/10.1656/1092-6194(2002)009[0267:ADIPPB]2.0.CO;2.
Elo M., Penttinen J., Kotiaho J.S. (2015). The effect of peatland drainage and restoration on Odonata species richness and abundance. BMC Ecology 15(11). http://dx.doi.org/10.1186/s12898-015-0042-z.
Eurola S., Huttunen A., Kukko-oja K. (1995). Suokasvillisuusopas. [Guide to mire vegetation]. Oulanka Reports 14. 85 p. [In Finnish].
European Commission (2011). Our life insurance, our natural capital: an EU biodiversity strategy to 2020. Communication from the commission to the European parliament, the council, the economic and social committee and the committee of the regions. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2011:0244:FIN:EN:PDF.
Fagan K.C., Pywell R.F., Bullock J.M., Marrs R.H. (2010). Are ants useful indicators of restoration success in temperate grasslands? Restoration Ecology 18: 373–379. http://dx.doi.org/10.1111/j.1526-100X.2008.00452.x.
Finér L., Jurgensen M.F., Domisch T., Kilpeläinen J., Neuvonen S., Punttila P., Risch A.C., Ohashi M., Niemelä P. (2013). The role of wood ants (Formica rufa group) in carbon and nutrient dynamics of a boreal Norway spruce forest ecosystem Ecosystems 16: 196–208. http://dx.doi.org/10.1007/s10021-012-9608-1.
Fowler H.G. (1987). Colonization patterns of the leaf-cutting ant, Atta bisphaerica Forel - Evidence for population regulation. Journal of Applied Entomology 104: 102–105. http://dx.doi.org/10.1111/j.1439-0418.1987.tb00503.x.
Franch J., Espadaler X. (1988). Ants as colonizing agents of pine stumps in San Juan de la Peña (Huesca, Spain). Vie et Milieu 38: 149–154.
Gösswald K. (1951). Versuche zum Sozialparasitismus der Ameisen bei der Gattung Formica L. [Experiments on the social parasitism of ants in the genus Formica]. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere 80: 533–582.
Gösswald K. (1952). Über Versuche zur Verwendung von Hilfameisen zwecks Vermehrung der nützlichen kleinen Roten Waldameise. [On experiments to use Serviformica ants in order to propagate the beneficial wood ant Formica polyctena]. Zeitschrift für Angewandte Entomologie 34: 1–44. http://dx.doi.org/10.1111/j.1439-0418.1953.tb00687.x.
Gotelli N.J., Ellison A.M. (2002a). Assembly rules for New England ant assemblages. Oikos 99: 591–599. http://dx.doi.org/10.1034/j.1600-0706.2002.11734.x.
Gotelli N.J., Ellison A.M. (2002b). Biogeography at a regional scale: determinants of ant species density in New England bogs and forests. Ecology 83: 1604–1609. http://dx.doi.org/10.1890/0012-9658(2002)083[1604:BAARSD]2.0.CO;2.
Gotelli N.J., Ellison A.M., Dunn R.R., Sanders N.J. (2011). Counting ants (Hymenoptera: Formicidae): biodiversity sampling and statistical analysis for myrmecologists. Myrmecological News 15: 13–19. http://myrmecologicalnews.org/cms/index.php?option=com_content&view=category&id=477:myrmecol-news-15-13-19&Itemid=85&layout=default.
Haapalehto T. (2014). Restoring ecosystem structure and functions: results from Sphagnum peatlands degraded by forestry drainage. Jyväskylä Studies in Biological and Environmental Science 275: 1–50. https://jyx.jyu.fi/dspace/handle/123456789/43088.
Haapalehto T.O., Vasander H., Jauhiainen S., Tahvanainen T., Kotiaho J.S. (2011). The effects of peatland restoration on water-table depth, elemental concentrations, and vegetation: 10 years of changes. Restoration Ecology 19: 587–598. http://dx.doi.org/10.1111/j.1526-100X.2010.00704.x.
Haapalehto T., Kotiaho J.S., Matilainen R., Tahvanainen T. (2014). The effects of long-term drainage and subsequent restoration on water table level and pore water chemistry in boreal peatlands. Journal of Hydrology 519: 1493–1505. http://dx.doi.org/10.1016/j.jhydrol.2014.09.013.
Hanski I. (2008). Insect conservation in boreal forests. Journal of Insect Conservation 12: 451–454. http://dx.doi.org/10.1007/s10841-007-9085-6.
Hanski I., Ovaskainen O. (2002). Extinction debt at extinction threshold. Conservation Biology 16: 666–673. http://dx.doi.org/10.1046/j.1523-1739.2002.00342.x.
Higgins R.J., Lindgren B.S. (2012). An evaluation of methods for sampling ants (Hymenoptera: Formicidae) in British Columbia, Canada. Canadian Entomologist 144: 491–507. http://dx.doi.org/10.4039/tce.2012.50.
Hölldobler B., Wilson E.O. (1990). The ants. Harvard University Press, Cambridge, Mass. 732 p. http://dx.doi.org/10.1007/978-3-662-10306-7.
Jauhiainen S., Laiho R., Vasander H. (2002). Ecohydrological and vegetational changes in a restored bog and fen. Annales Botanici Fennici 39: 185–199. http://www.sekj.org/PDF/anbf39/anbf39-185p.pdf.
Kaakinen E., Kokko A., Aapala K., Kalpio S., Eurola S., Haapalehto T., Heikkilä R., Hotanen J.-P., Kondelin H., Nousiainen H., Ruuhijärvi R., Salminen P., Tuominen S., Vasander H., Virtanen K. (2008). Suot. [Mires]. In: Raunio A., Schulman A., Kontula T. (eds.). Suomen luontotyyppien uhanalaisuus - osa 1: tulokset ja arvioinnin perusteet. [Assessment of threatened habitat types in Finland - part 1: results and basis for assessment]. Suomen ympäristökeskus, Suomen ympäristö 8/2008. [Finnish Environment Institute, The Finnish Environment 8/2008]. p. 75–109. https://helda.helsinki.fi/handle/10138/37930?locale-attribute=en.
Kilpeläinen J., Punttila P., Finér L., Niemelä P., Domisch T., Jurgensen M.F., Neuvonen S., Ohashi M., Risch A.C., Sundström L. (2008). Distribution of ant species and mounds (Formica) in different-aged managed spruce stands in eastern Finland. Journal of Applied Entomology 132: 315–325. http://dx.doi.org/10.1111/j.1439-0418.2007.01244.x.
Komulainen V.M., Tuittila E.-S., Vasander H., Laine J. (1999). Restoration of drained peatlands in southern Finland: initial effects on vegetation change and CO2 balance. Journal of Applied Ecology 36: 634–648. http://dx.doi.org/10.1046/j.1365-2664.1999.00430.x.
Kotiaho J.S., Moilanen A. (2015). Conceptual and operational perspectives on ecosystem restoration options in the European Union and elsewhere. Journal of Applied Ecology 52(4): 816–819. http://dx.doi.org/10.1111/1365-2664.12411.
Kotiaho J.S., Halme P., Kareksela S., Oldén A., Haapalehto T., Päivinen J., Moilanen A. (2015). Finland: target for ecosystem repair is impractical. Nature 519(33). http://dx.doi.org/10.1038/519033a.
Krogerus R. (1960). Ökologische Studien über nordische Moorarthropoden. [Ecological studies on Nordic mire arthropods]. Commentationes Biologicae Societas Scientiarum Fennica 21: 1–238.
Kutter H. (1969). Die sozialparasitischen Ameisen der Schweiz. [The socially parasitic ants of Switzerland]. Neujahrsblatt Herausgegeben von der Naturforschenden Gesellschaft in Zürich 171: 1–62.
Kuuluvainen T., Aapala K., Ahlroth P., Kuusinen M., Lindholm T., Sallantaus T., Siitonen J., Tukia H. (2002). Principles of ecological restoration of boreal forested ecosystems: Finland as an example. Silva Fennica 36(1): 409–422. http://dx.doi.org/10.14214/sf.572.
Laiho R., Silvan N., Cárcamo H., Vasander H. (2001). Effects of water level and nutrients on spatial distribution of soil mesofauna in peatlands drained for forestry in Finland. Applied Soil Ecology 16: 1–9. http://dx.doi.org/10.1016/S0929-1393(00)00103-7.
Laine J., Vasander H., Sallantaus T. (1995a). Ecological effects of peatland drainage for forestry. Environmental Reviews 3: 286–303. http://dx.doi.org/10.1139/a95-015.
Laine J., Vasander H., Laiho R. (1995b). Long-term effects of water level drawdown on the vegetation of drained pine mires in southern Finland. Journal of Applied Ecology 32: 785–802. http://dx.doi.org/10.2307/2404818.
Laine A.M., Leppälä M., Tarvainen O., Päätalo M.-L., Seppänen R., Tolvanen A. (2011). Restoration of managed pine fens: effect on hydrology and vegetation. Applied Vegetation Science 14: 340–349. http://dx.doi.org/10.1111/j.1654-109X.2011.01123.x.
Mabelis A.A., Chardon J.P. (2005). Survival of the Black bog ant (Formica transkaucasica Nasanov) in relation to the fragmentation of its habitat. Journal of Insect Conservation 9: 95–108. http://dx.doi.org/10.1007/s10841-004-5987-8.
Melbourne B.A. (1999). Bias in the effect of habitat structure on pitfall traps: an experimental evaluation. Australian Journal of Ecology 24: 228–239. http://dx.doi.org/10.1046/j.1442-9993.1999.00967.x.
Niemelä J., Haila Y., Punttila P. (1996). The importance of small-scale heterogeneity in boreal forests: variation in diversity in forest-floor invertebrates across the succession gradient. Ecography 19: 352–368. http://dx.doi.org/10.1111/j.1600-0587.1996.tb00246.x.
Noreika N., Kotiaho J.S., Penttinen J., Punttila P., Vuori A., Pajunen T., Autio O., Loukola O.J., Kotze D.J. (2015). Rapid recovery of invertebrate communities after ecological restoration of boreal mires. Restoration Ecology 23(5): 566–579. http://dx.doi.org/10.1111/rec.12237.
Oinonen E.A. (1956). Kallioiden muurahaisista ja niiden osuudesta kallioiden metsittymiseen Etelä-Suomessa. [On the ants of the rocks and their contribution to the afforestation of rocks in southern Finland]. Acta Entomologica Fennica 12: 1–212. [In Finnish with an English Summary]. p. 179–208.
Oinonen E., Wuorenrinne H. (1976). Über die finnischen Ameisenarten aus waldpflegerischen Gesichtspunkten. [On silvicultural aspects of the Finnish ant species]. Waldhygiene 11: 167–170.
Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H., Wagner H. (2013). vegan: community ecology package. R package version 2.0-8. http://CRAN.R-project.org/package=vegan.
Palmer M.A., Ambrose R.F., Poff N.L. (1997). Ecological theory and community restoration ecology. Restoration Ecology 5: 291–300. http://dx.doi.org/10.1046/j.1526-100X.1997.00543.x.
Peltola A. (ed.) (2014). Metsätilastollinen vuosikirja 2014. [Finnish Statistical Yearbook of Forestry]. Suomen virallinen tilasto: maa-, metsä- ja kalatalous 2014. [Official statistics of Finland: agriculture, forestry and fishery]. Metsäntutkimuslaitos. [Finnish Forest Research Institute]. 428 p. http://www.metla.fi/metinfo/tilasto/julkaisut/vsk/2014/.
Persson T., Lenoir L., Vegerfors B. (2013). Which macroarthropods prefer tree stumps over soil and litter substrates? Forest Ecology and Management 290: 30–39. http://dx.doi.org/10.1016/j.foreco.2012.09.009.
Pontin A.J. (1960). Field experiments on colony foundation by Lasius niger (L.) and L. flavus (F.) (Hym., Formicidae). Insectes Sociaux 7(3): 227–230. http://dx.doi.org/10.1007/BF02224494.
Punttila P. (1996). Succession, forest fragmentation, and the distribution of wood ants. Oikos 75: 291–298. http://dx.doi.org/10.2307/3546252.
Punttila P., Haila Y. (1996). Colonisation of a burned forest by ants in the southern Finnish boreal forest. Silva Fennica 30(4): 421–435. http://dx.doi.org/10.14214/sf.a8502.
Punttila P., Kilpeläinen J. (2009). Distribution of mound-building ant species (Formica spp., Hymenoptera) in Finland: preliminary results of a national survey. Annales Zoologici Fennici 46: 1–15. http://dx.doi.org/10.5735/086.046.0101.
Punttila P., Haila Y., Pajunen T., Tukia H. (1991). Colonisation of clearcut forests by ants in the southern Finnish taiga: a quantitative survey. Oikos 61: 250–262. http://dx.doi.org/10.2307/3545343.
Punttila P., Haila Y., Niemelä J., Pajunen T. (1994). Ant communities in fragments of old-growth taiga and managed surroundings. Annales Zoologici Fennici 31: 131–144. http://www.sekj.org/PDF/anzf31/anz31-131-144.pdf.
Punttila P., Haila Y., Tukia H. (1996). Ant communities in taiga clearcuts: habitat effects and species interactions. Ecography 19: 16–28. http://dx.doi.org/10.1111/j.1600-0587.1996.tb00151.x.
Punttila P., Vepsäläinen K., Väänänen S. (2013). Muurahaiset, soiden ojitus ja ennallistaminen. [Ants, mire drainage and restoration]. In: Aapala K., Similä M., Penttinen J. (eds.). Ojitettujen soiden ennallistamisopas. [Handbook for the restoration of drained peatlands]. Metsähallitus. Metsähallituksen luonnonsuojelujulkaisuja. Sarja B 188. p. 86–90. [In Finnish with an English summary]. https://julkaisut.metsa.fi/julkaisut/show/1601.
R Core Team. (2013). R: a language and environment for statistical computing. Version 3.0.1. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org/.
Rassi P., Alanen A., Kanerva T., Mannerkoski I. (eds.). (2001). Suomen lajien uhanalaisuus 2000. [The 2000 red list of finnish species]. Ympäristöministeriö & Suomen ympäristökeskus. [Ministry of the Environment & Finnish Environment Institute]. 432 p. [In Finnish with an English summary].
Rassi P., Hyvärinen E., Juslén A., Mannerkoski I. (eds.) (2010). Suomen lajien uhanalaisuus - punainen kirja 2010. [The 2010 red list of finnish species]. Ympäristöministeriö ja Suomen ympäristökeskus. [Ministry of the Environment and Finnish Environment Institute]. 685 p. http://www.ymparisto.fi/en-US/Nature/Species/Threatened_species/The_2010_Red_List_of_Finnish_species.
Ratchford J.S., Wittman S.E., Jules E.S., Ellison A.M., Gotelli N.J., Sanders N.J. (2005). The effects of fire, local environment and time on ant assemblages in fens and forests. Diversity and Distributions 11: 487–497. http://dx.doi.org/10.1111/j.1366-9516.2005.00192.x.
Raunio A., Schulman A., Kontula T. (eds.) (2008). Suomen luontotyyppien uhanalaisuus - osa 2: luontotyyppien kuvaukset. [Assessment of theatened habitat types in Finland - part 2: habitat type descriptions]. Suomen ympäristökeskus, Suomen ympäristö 8/2008, osa 2. 572 p. [In Finnish with an English summary]. https://helda.helsinki.fi/handle/10138/37932.
Rees S.D., Orledge G.M., Bruford M.W., Bourke A.F.G. (2010). Genetic structure of the Black Bog Ant (Formica picea Nylander) in the United Kingdom. Conservation Genetics 11: 823–834. http://dx.doi.org/10.1007/s10592-009-9915-z.
Ruuhijärvi R. (1983). The Finnish mire types and their regional distribution. In: Gore A.J.P. (ed.). Ecosystems of the world: 4B. Mires: Swamp, Bog, Fen and Moor. Regional studies. Elsevier. p. 47–67.
Rydin H., Jeglum J. (2006). The biology of peatlands. Oxford University Press, New York. 343 p. http://dx.doi.org/10.1093/acprof:oso/9780198528722.001.0001.
Sanders N.J., Gotelli N.J., Wittman S.E., Ratchford J.S., Ellison A.M., Jules E.S. (2007). Assembly rules of ground-foraging ant assemblages are contingent on disturbance, habitat and spatial scale. Journal of Biogeography 34: 1632–1641. http://dx.doi.org/10.1111/j.1365-2699.2007.01714.x.
Schielzeth H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution 1: 103–113. http://dx.doi.org/10.1111/j.2041-210X.2010.00012.x.
Seifert B. (2000). A taxonomic revision of the ant subgenus Coptoformica Mueller, 1923 (Hymenoptera: Formicidae). Zoosystema 22: 517–568.
Seifert B. (2007). Die Ameisen Mittel- und Nordeuropas. [The ants of Central and Northern Europe]. Lutra Verlags- und Vetriebsgesellschaft, Görlitz/Tauer. 368 p.
Seppä P. (2008). Do ants (Hymenoptera: Formicidae) need conservation and does ant conservation need genetics? Myrmecological News 11: 161–172. http://myrmecologicalnews.org/cms/index.php?option=com_content&view=category&id=255:myrmecol-news-11-161-172&Itemid=73&layout=default.
Seppä P., Sundström L., Punttila P. (1995). Facultative polygyny and habitat succession in boreal ants. Biological Journal of the Linnean Society 56: 533–551. http://dx.doi.org/10.1111/j.1095-8312.1995.tb01109.x.
Similä M., Aapala K., Penttinen J. (eds.). (2014). Ecological restoration in drained peatlands - best practices from Finland. Metsähallitus, Natural Heritage Services and Finnish Environment Institute SYKE. 84 p. http://julkaisut.metsa.fi/julkaisut/show/1733.
Society for Ecological Restoration International Science & Policy Working Group (2004). The SER international primer on ecological restoration. Society for Ecological Restoration International, Washington D.C. 15 p. http://www.ser.org/resources/resources-detail-view/ser-international-primer-on-ecological-restoration.
Soro A., Sundberg S., Rydin H. (1999). Species diversity, niche metrics and species associations in harvested and undisturbed bogs. Journal of Vegetation Science 10: 549–560. http://dx.doi.org/10.2307/3237189.
Sorvari J., Hakkarainen H. (2005). Deforestation reduces nest mound size and decreases the production of sexual offspring in the wood ant Formica aquilonia. Annales Zoologici Fennici 42: 259–267. http://www.sekj.org/PDF/anzf42/anzf42-259.pdf.
Sorvari J., Hakkarainen H. (2007). Wood ants are wood ants: deforestation causes population declines in the polydomous wood ant Formica aquilonia. Ecological Entomology 32: 707–711. http://dx.doi.org/10.1111/j.1365-2311.2007.00921.x.
Sorvari J., Haatanen M.-K., Vesterlund S.-R. (2011). Combined effects of overwintering temperature and habitat degradation on the survival of boreal wood ant. Journal of Insect Conservation 15: 727–731. http://dx.doi.org/10.1007/s10841-010-9372-5.
Spitzer K., Danks H.V. (2006). Insect biodiversity of boreal peat bogs. Annual Review of Entomology 51: 137–161. http://dx.doi.org/10.1146/annurev.ento.51.110104.151036.
Sundström L., Seppä P., Pamilo P. (2005). Genetic population structure and dispersal patterns in Formica ants - a review. Annales Zoologici Fennici 42: 163–177. http://www.sekj.org/PDF/anzf42/anzf42-163.pdf.
Sveum P. (1978). On the biology of ants (Hymenoptera, Formicidae) in Trøndelag, Norway. Norwegian Journal of Entomology 25: 153–155.
Tilman D., May R.M., Lehman C.L., Nowak M.A. (1994). Habitat destruction and the extinction debt. Nature 371: 65–66. http://dx.doi.org/10.1038/371065a0.
Tschinkel W.R. (1987). The fire ant, Solenopsis invicta, as a successful ‘weed’. In: Eder J., Rembold H. (eds.). Chemistry and biology of social insects. Verlag J. Peperny. p. 585–588.
Underwood E.C., Fisher B.L. (2006). The role of ants in conservation monitoring: if, when, and how. Biological Conservation 132: 166–182. http://dx.doi.org/10.1016/j.biocon.2006.03.022.
Uusitalo A., Kotiaho J.S., Päivinen J., Rintala T., Saari V. (2006). Kasvien ja päiväperhosten esiintyminen luonnontilaisilla ja ojitetuilla soilla. [Distribution of plants and butterflies in natural and drained peatlands]. Metsähallituksen luonnonsuojelujulkaisuja sarja A 157. [Nature Protection Publications of Metsähallitus, Series A 157]. 44 p. [In Finnish with an English summary]. http://julkaisut.metsa.fi/julkaisut/show/207.
Uuttera J., Maltamo M., Hotanen J.-P. (1997). The structure of forest stands in virgin and managed peatlands: a comparison between Finnish and Russian Karelia. Forest Ecology and Management 96: 125–138. http://dx.doi.org/10.1016/S0378-1127(97)00035-2.
van Duinen G.-J.A., Brock A.M.T., Kuper J.T., Leuven R.S.E.W., Peeters T.M.J., Roelofs J.G.M., van der Velde G., Verberk W.C.E.P., Esselink H. (2003). Do restoration measures rehabilitate fauna diversity in raised bogs? A comparative study on aquatic macroinvertebrates. Wetlands Ecology and Management 11: 447–459. http://dx.doi.org/10.1023/B:WETL.0000007196.75248.a5.
Vasander H., Tuittila E.-S., Lode E., Lundin L., Ilomets M., Sallantaus T., Heikkilä R., Pitkänen M.-L., Laine J. (2003). Status and restoration of peatlands in northern Europe. Wetlands Ecology and Management 11: 51–63. http://dx.doi.org/10.1023/A:1022061622602.
Watts C.H., Clarkson B.R., Didham R.K. (2008). Rapid beetle community convergence following experimental habitat restoration in a mined peat bog. Biological Conservation 141: 568–579. http://dx.doi.org/10.1016/j.biocon.2007.12.008.
Vepsäläinen K., Pisarski B. (1982). Assembly of island ant communities. Annales Zoologici Fennici 19: 327–335. http://www.sekj.org/PDF/anzf19/anz19-327-335.pdf.
Vepsäläinen K., Savolainen R., Tiainen J., Vilén J. (2000). Successional changes of ant assemblages: from virgin and ditched bogs to forests. Annales Zoologici Fennici 37: 135–149. http://www.sekj.org/PDF/anzf37/anzf37-135p.pdf.
Wilson E.O. (1971). The insect societies. Harvard University Press, Cambridge, Mass. 548 p.
Wilson E.O., Hunt G.L., Jr. (1966). Habitat selection by the queens of two field-dwelling species of ants. Ecology 47: 485–487. http://dx.doi.org/10.2307/1932989.
Włodarczyk T., Żmihorski M., Olczyk A. (2009). Ants inhabiting stumps on clearcuts in managed forest in western Poland. Entomologica Fennica 20: 121–128.
Working Group on a National Strategy for Mires and Peatlands (2011). Proposal for a national strategy for the sustainable and responsible use of mires and peatlands. Working group memorandum MMM 2011:1. Ministry of Agriculture and Forestry. 161 p. [In Finnish with an English summary].
Total of 109 references.
