Influence of climate and forest management on damage risk by the pine weevil Hylobius abietis in northern Sweden
Nordlander G., Mason E. G., Hjelm K., Nordenhem H., Hellqvist C. (2017). Influence of climate and forest management on damage risk by the pine weevil Hylobius abietis in northern Sweden. Silva Fennica vol. 51 no. 5 article id 7751. https://doi.org/10.14214/sf.7751
Highlights
- Analysis of survey data from 292 reforestation areas in northern Sweden show that the probability of pine weevil damage can be predicted with a standard error of 0.12
- Three variables are important in the optimal model: proportion of seedlings in mineral soil, age of clear-cut, and temperature sum
- Temperature sum in the model can be adjusted to reflect future climate scenarios.
Abstract
The pine weevil Hylobius abietis L. is an economically important pest insect that kills high proportions of conifer seedlings in reforestation areas. It is present in conifer forests all over Europe but weevil abundance and risk for damage varies considerably between areas. This study aimed to obtain a useful model for predicting damage risks by analyzing survey data from 292 regular forest plantations in northern Sweden. A model of pine weevil attack was constructed using various site characteristics, including both climatic factors and factors related to forest management activities. The optimal model was rather imprecise but showed that the risk of pine weevil attack can be predicted approximatively with three principal variables: 1) the proportion of seedlings expected to be planted in mineral soil rather than soil covered with duff and debris, 2) age of clear-cut at the time of planting, and 3) calculated temperature sum at the location. The model was constructed using long-run average temperature sums for epoch 2010, and so effects of climate change can be inferred from the model by adjustment to future epochs. Increased damage risks with a warmer climate are strongly indicated by the model. Effects of a warmer climate on the geographical distribution and abundance of the pine weevil are also discussed. The new tool to better estimate the risk of damage should provide a basis for foresters in their choice of countermeasures against pine weevil damage in northern Europe.
Keywords
temperature sum;
reforestation;
soil scarification;
clear-cut age;
conifer seedling;
damage prediction;
warmer climate
- Nordlander, Swedish University of Agricultural Sciences (SLU), Department of Ecology, P.O. Box 7044, SE-750 07 Uppsala, Sweden E-mail Goran.Nordlander@slu.se
-
Mason,
University of Canterbury, School of Forestry, Private Bag 4800, Christchurch 8140, New Zealand; Swedish University of Agricultural Sciences, Southern Swedish Forest Research Centre, P.O. Box 49, SE-230 53 Alnarp, Sweden
 http://orcid.org/0000-0001-9024-9106
E-mail
euan.mason@canterbury.ac.nz
http://orcid.org/0000-0001-9024-9106
E-mail
euan.mason@canterbury.ac.nz

- Hjelm, Skogforsk, The Forest Research Institute of Sweden, Ekebo 2250, SE-268 90 Svalöv, Sweden; Swedish University of Agricultural Sciences, Southern Swedish Forest Research Centre, P.O. Box 49, SE-230 53 Alnarp, Sweden E-mail karin.hjelm@skogforsk.se
- Nordenhem, Swedish University of Agricultural Sciences (SLU), Department of Ecology, P.O. Box 7044, SE-750 07 Uppsala, Sweden E-mail h.nordenhem@telia.com
- Hellqvist, Swedish University of Agricultural Sciences (SLU), Department of Ecology, P.O. Box 7044, SE-750 07 Uppsala, Sweden E-mail Claes.Hellqvist@slu.se
Received 13 June 2017 Accepted 12 October 2017 Published 20 October 2017
Views 190545
Available at https://doi.org/10.14214/sf.7751 | Download PDF

1 Introduction
The current global warming will lead to an increase in temperature of several degrees C in northern Europe during the next 100 years, and the largest temperature increases are found in northern Fennoscandia (Kjellström et al 2011; Bärring et al. 2017). A warmer climate in the Boreal zone of Europe is mostly expected to positively affect forest growth and wood production (Jansson et al. 2008; Ge et al. 2013) but, on the other hand, also increase the risk of damage by various forest pests (Logan et al. 2003; Lindner et al. 2010). Direct responses to higher temperatures in phytophagous insects may enhance their reproductive potential and lead to higher abundance and more feeding causing damage to their hosts (Bale et al. 2002; Netherer and Schopf 2010). Responses by host trees and by natural enemies to higher temperatures and interactions between trophic levels may, however, lead also to other outcomes than increased pest problems (Tylianakis et al. 2008; Berggren et al. 2009; Baffoe et al. 2012). The extent and severity of damage by many forest insect pests are strongly influenced by forest management (Jactel et al. 2009), and adaptation measures need to be implemented in forestry to meet the challenges of climate change (Ayres and Lombardero 2000; Kolström et al. 2011; Björkman et al. 2015; Keskitalo et al. 2016; Subramanian et al. 2016). Management adaptations may be particularly urgent for forest regeneration practices (Nilsson et al. 2010; Kolström et al. 2011), not the least as a tool in integrated pest management of economically important regeneration pests (Nordlander et al. 2011).
The most destructive forest regeneration pest in large parts of Europe and Asia is the pine weevil Hylobius abietis L. (Långström and Day 2004). The adult weevils cause the damage by feeding on the stem bark of planted conifer seedlings, which are frequently killed by girdling near the stem base (Day et al. 2004). Similar damage is caused also by Hylobius pinastri Gyll., a less frequent species preferring moist sites with Norway spruce (Långström 1982; Viiri and Miettinen 2013). If no countermeasures are taken the seedling mortality may, e.g. in southern Sweden, reach levels up to almost 100% (von Sydow 1997). Annual costs in Sweden for the damage have been estimated to approximately 30 million USD (Mattsson 2016). In European conifer forests managed by clear-felling and planting, the pine weevil problem is still to a large extent controlled by insecticide treatment of the seedlings prior to planting (Långström and Day 2004). Alternatives to replace the insecticides have long been sought and the use of coatings on the stem for physical protection of seedlings is rapidly increasing in Sweden (Nordlander et al. 2009; Giurca and von Stedingk 2014; Skogsstyrelsen 2017). Direct protection of seedlings is, however, not the sole measure against the pine weevil. Damage can often be reduced to low levels by combining a number of management measures, or combining them with direct seedling protection in high risk areas (Petersson and Örlander 2003; Nordlander et al. 2011). Such integrated pest management measures may include planting in mineral soil after soil scarification, use of seedlings with relatively thick stems, use of an appropriate fallow period, and planting under shelterwood (von Sydow 1997; Örlander and Nilsson 1999; Nordlander et al. 2003, 2011, 2017).
Choice of appropriate countermeasures against pine weevil damage can be greatly helped by predictions of damage risk levels. Weevil damage intensity varies considerably between sites but causes of this variation remain insufficiently known (Wilson et al. 1996; Hansen et al. 2005; Luoranen et al. 2017). Some important factors have though been identified, for example soil condition at planting spots, age of clear-cut, and climate conditions (as described below).
Site preparation that exposes mineral soil in which the seedlings are planted is a common practice in northern Europe (Nilsson et al. 2010) which drastically reduces pine weevil damage compared to planting directly in the humus (Lindström et al. 1986; von Sydow 1997; Örlander and Nilsson 1999; Peterson et al. 2005; Nordlander et al. 2005, 2011; Wallertz and Petersson 2011; Luoranen et al. 2017). Pine weevils move more rapidly on mineral soil than on humus (Kindvall et al. 2000) and the presence of pure mineral soil around a seedling strongly reduces the likelihood that an approaching pine weevil will feed on it (Björklund et al. 2003). Although the strong damage-reducing effect of soil preparation is closely linked to planting in exposed mineral soil, soil preparation on peatland also has been shown to decrease damage by pine weevil (Luoranen and Viiri 2012).
The age of a clear-cut at the time of planting strongly influences the risk for damage by feeding pine weevils (von Sydow 1997; Örlander and Nilsson 1999; Wallertz et al. 2016; Luoranen et al. 2017; Nordlander et al. 2017). Generally, damage risk is high during the first three growing seasons after harvest. During the first season after harvest of a coniferous stand, pine weevils invade a clear-cut in high numbers during their migration flight in late spring (Solbreck and Gyldberg 1979). The weevils are attracted by odours from the fresh stumps, since the stump roots are used as breeding substrate. The parent weevils feed on bark and phloem of conifer seedlings but also on roots and twigs of larger trees (Wallertz et al. 2006). They remain on a clear-cut during the entire summer and, after hibernation, also the following season. Then the new generation of weevils usually emerges in late summer, and they remain feeding at the site both during autumn and spring the following year (i.e., third season after harvest), after which they fly away to new breeding sites in late spring (this applies to regions with a 2-year life cycle; see below).
Populations of H. abietis are adapted to a wide range of climatic conditions. The species is present all over Europe wherever there are conifer trees that can be used as hosts (Långström and Day 2004). In northern Europe, the risk of damage by the pine weevil is generally considered to be lower in locations with a colder climate (Långström 1985; Nordlander et al. 2011), but little is actually known about how geographical variation in temperature conditions influences damage levels. Temperature conditions strongly affect time for development, resulting in a life cycle duration of usually 2 years but only 1 year in the warmer areas and up to 4 years in the coldest (Bejer-Petersen et al. 1962; Inward et al. 2012; Wainhouse et al.2014). The amount of time over the year with ambient temperatures suitable for pine weevil feeding probably has even more influence on damage levels (Tan et al. 2010; Björkman et al. 2015). Thus, in northern Europe damage by pine weevil should decrease with higher latitude and altitude since the cooler climate in these areas decreases the number of days with feeding activity in spring and autumn.
In northern Sweden (approximately N of latitude 61°N) previously reported damage levels (averaged over several sites) varied between almost zero and around 25% for unprotected seedlings planted after soil scarification (Nordlander et al. 2011; Johansson et al. 2015). In this region, approximately 90% of seedlings are not protected against pine weevil damage by treatment with insecticide or stem coating, and the pine weevil problem is primarily handled by a combination of silvicultural measures. For this reason there is a very high demand in northern Sweden for damage risk estimates as a basis for choosing cost-effective combinations of countermeasures. Risk can be estimated by examining proportions of seedlings damaged under any specific set of circumstances, and so a model of proportions is also a model of damage risk if the circumstances are well characterized as independent variables in the model.
The objective of this study was to develop a model for predicting risks of damage by the pine weevil in reforestation areas in northern Sweden. A useful relative measure was the goal, not absolute estimates of final seedling mortality. To this end we surveyed the damage on seedlings after one season in the field in forest plantations all over northern Sweden and registered various site characteristics, including calculated temperature sums. A model was constructed to analyze these data so that expected damage risks in northern Sweden could be presented in a series of maps. Since the damage risks presented are highly related to temperature sums, future changes in damage levels caused by a warmer climate are considered.
2 Materials and methods
2.1 Sites and site characteristics
A survey of damage by the pine weevil (Hylobius abietis) on planted conifer seedlings was conducted over six years between 2006 and 2011 in forests regenerated after clearfelling by forest companies or private forest owners (Table 1). A total of 292 plantations were surveyed over those years, distributed throughout northern Sweden east of the Scandinavian Mountains down to the coast line between latitude 61°N and the polar circle (66°34´N) (Fig. 1). The minimum distance between sites was 1 km. Three criteria had to be fulfilled for inclusion in the survey:
1) Plantations should have been established during the spring or early summer of the same year that the inventory was conducted during autumn.
2) Seedlings planted had to be of one of the three conifer species predominantly used in northern Sweden, Norway spruce (Picea abies (L.) Karst.), Scots pine (Pinus sylvestris L.) or lodgepole pine (Pinus contorta Douglas ex Loudon).
3) The seedlings were not protected against pine weevil damage with insecticides or any physical protection.
| Table 1. Numbers of sites inventoried in each year by age since clearfelling (1 year old = 2nd season after harvesting). | ||||
| Year | 1 year old | 2 year old | 3 year old | Total |
| 2006 | 2 | 21 | 3 | 26 |
| 2007 | 2 | 13 | 10 | 25 |
| 2008 | 7 | 18 | 8 | 33 |
| 2009 | 5 | 53 | 30 | 87 |
| 2010 | 22 | 48 | 23 | 93 |
| 2011 | 5 | 6 | 16 | 27 |
| Total | 43 | 158 | 89 | 292 |
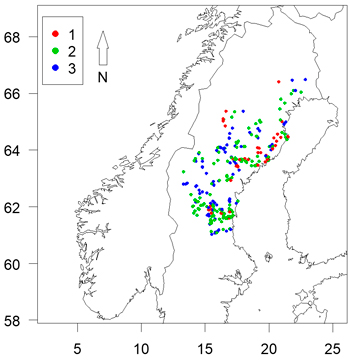
Fig. 1. Locations of sites assessed coloured by clear-cut age. Axes show longitude and latitude.
Sites were chosen with regard to relative ease of access in order to maximize the number of sites that could be visited during the limited time the inventories had to be conducted (a few weeks in autumn after completed damage and before risk for snowfall). Because time was limited, a number of sites within the same region were visited before moving on elsewhere. The practical limitations and the fulfillment of the criteria mentioned above made the material unbalanced in some aspects (see the Results section), but in general the surveyed plantations were considered to be representative of northern Sweden.
Site characteristics, both in terms of climatic factors and factors related to forest management activities, were collected by inspection at the sites or based on information obtained from the forest owner (Table 2). Among the sites, the age of the clear-cut differed at time of planting, reaching from 1 year old (i.e. 2nd season after harvesting) to 3 year old clear-cuts (Table 1). Temperature sum (TS), defined as the sum of the daily mean temperature above +5 °C during one growing season, for each site was calculated by Eq. 1:
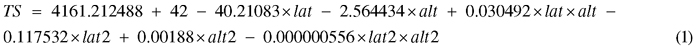
where lat = latitud (°N) and alt = altitud (m). Eq. 1 is derived from Odin et al. (1983) and has been adjusted to correspond to average climate prevailing in the 2010 epoch (Berlin et al. 2014).
| Table 2. Potential independent variables. | |
| Variable | Range or categories |
| Coordinate RT90* East/West | 1373546 to 1813800 |
| Coordinate RT90 North/South | 6767903 to 7393400 |
| Coast distance | 1 to 240 km |
| Elevation | 7 to 660 m above mean sea level |
| Temperature sum | 548.9 to 1267.7 degree days above 5 °C per year |
| Clear-cut age | 1, 2 or 3 years |
| Site index | T14 to T24 for pine and G14 to G28 for spruce (height in m after 100 years) |
| Tree species | Norway spruce, Scots pine or lodgepole pine |
| Stoniness | None, light, medium or heavy |
| Site preparation | Disc trenching, patch scarification or mounding |
| Mineral soil on site | 0% to 88% of planted seedlings surrounded by pure mineral soil |
| * RT90 is a coordinate reference system with the following prog4string: +proj=tmerc +lat_0=0 +lon_0=15.80827777777778 +k=1 +x_0=1500000 +y_0=0 +ellps=bessel +units=m +no_defs | |
2.2 Damage assessment
Inventories were made during autumn after the seedlings had been exposed in the field for one season. At this time it was still possible to locate almost all planted seedlings, even those that had died soon after planting, leaving only part of the debarked stem and the peat clump underground. On each site 20 circular sample plots with an area of 20 m2 (radius 2.52 m) were established along an angled transect over the plantation with a fixed distance between plots. This fixed distance was set longer for larger plantations and shorter for the smaller ones (minimum distance 15 m), in order to get a good representation of each site. The transect was laid out with some angles so that it reached different parts of the site, but it was not laid over parts that had not been planted at all (e.g., patches with retained trees, bare rocks, very wet parts). The entire area of each sample plot was carefully searched for living and dead planted seedlings; naturally regenerated seedlings were not included. An average number of 86 planted seedlings (range 42–148) were found in the sampling plots at each site and these were assessed for vitality and feeding damage by pine weevil or damage caused by other factors. Feeding scars by pine weevil (H. abietis or, to a less extent, H. pinastri) on the stem bark were recorded with 0.1 cm2 as the minimum feeding area recorded, and it was noted if the stem was completely girdled. To describe the detrimental effects on seedlings by the pine weevil, the following classifications were made:
Attacked seedlings – the proportion of seedlings with any scar (minimum size 0.1 cm) on the stem judged to be caused by pine weevil feeding.
Mortality by pine weevil – the proportion of seedlings considered to be killed by pine weevil feeding, including both dead and still alive seedlings that were girdled on the lower part of the stem.
Each planting spot was classified into one of four categories according to the state of the soil within a radius of 10 cm around the seedling: (1) undisturbed humus, (2) cultivated humus (≤10% mineral soil), (3) humus/mineral soil mixture (>10% but ≤90% mineral soil), and (4) pure mineral soil (>90% mineral soil).
In addition to the main inventory we collected some data regarding the amount of damage added during the second season. The seedlings on 27 sites were marked with sticks on the first inspection and then revisited one year later. For these sites also the total mortality after two seasons was recorded, i. e. mortality by pine weevil and all other causes.
2.3 Statistical analysis
A model of pine weevil attack was constructed using known impacts on weevils; proportion of planting spots with pure mineral soil and clear-cut age, and by including several factors related to the site (Table 2). Each site was a sampling unit, and plots were averaged across each site to arrive at estimates of damage with large numbers of seedlings.
Distributions of variables were plotted, and it was clear that many were non-normal. Scaled power transformations (Eq. 2) were employed to make them as normal as possible before analysis:

where χ is the variable being transformed and λ is a coefficient that varies typically between –3 and 5 (Cook & Weisberg 1999).
Previous studies evaluating risk have often used logistic regression to model binary data (Wilson et al. 1996; Selander et al. 1990) or in one case Cox proportional hazards regression (Selander 1993). Logistic regression requires each sampling unit (tree) to be independent, and this was not so in our study. Cox proportional hazards regression assumes effects are constant over time and additive. In our design these assumptions were also open to question. Instead, we chose to use multiple linear regression, transforming proportions of seedlings influenced by weevils in order to make them as normal as possible. Hence the sampling unit was site, and sampling units were independent, satisfying a critical assumption of multilinear regression.
The square root of the number of plants assessed at each site was used as a weight in order to partially account for differences in sample size when estimating model coefficients. Tests of relationships between model residuals and other variables were then used to select additional variables for inclusion in the model, bearing in mind multi-collinearity and testing for interactions. Modelling began with all possible terms and interactions, and then least significant terms were sequentially removed, one by one, and the model was recomputed at each step. Only significant effects were retained, except where main effects were required in order to allow for the principle of marginality. Where necessary, alternative models were compared using the anova command in R (R Development Core Team 2004), and tests of normality of residuals and plots of residuals versus predicted values were used to assess goodness of fit. After the optimal multi-linear model was chosen, a mixed effects model using year as a random effect was fitted and compared with the regression model using Akaike’s information criterion (AIC) (Akaike 1978).
Survey data are almost always unbalanced, and this was particularly so for data from 1 year old clear-cuts (Fig. 1). In order to test for the effects of imbalance, the analysis was repeated using only data from 2 and 3 year old clear-cuts.
Models of proportions often have awkward residuals, and so an additional analysis was tried using a logistic regression to model the likelihood of any weevil attack occurring, and then combining that with a model of the proportion of seedlings attacked made using data from sites where attack was non-zero. A similar approach has been developed for modelling mortality of older trees in permanent sample plots (Woollons 1998). The combined model was compared with the single step one by computing the residual sums of squares of each approach and choosing the method that yielded the lowest residual.
The proportion of attacked seedlings that died was modelled using data from plots where at least 20% of seedlings had been attacked, using the number of attacked seedlings as a weight. Statistical procedures and assessments were the same as for the model of seedlings attacked.
3 Results
There was a high level of potential multi-collinearity in the dataset (Table 3). It is clear from Fig. 1 that the sample was unbalanced, with a high number of 1 year old clear-cuts located near the coast and to the north of the sample area. As clear-cut age affects the likelihood of damage, this limited our ability to assess the impacts of spatial variables on likelihood of damage.
| Table 3. Simple correlations between variables. Numbers in bold are statistically significant. | |||||||||
| Prob. attack | Clear-cut age | Mineral soil | Elevation | Coast distance | Temp. sum | Coordinate E/W | Coordinate N/S | Stoniness | |
| Prob. attack | 1 | –0.14226 | –0.39653 | –0.40913 | –0.43813 | 0.418602 | 0.279088 | –0.08671 | 0.158614 |
| Clear-cut age | –0.14226 | 1 | 0.098893 | 0.137607 | 0.218259 | –0.07446 | –0.22084 | –0.09351 | –0.01117 |
| Mineral soil | –0.39653 | 0.098893 | 1 | 0.354485 | 0.395688 | –0.41492 | –0.18979 | 0.168581 | –0.15065 |
| Elevation | –0.40913 | 0.137607 | 0.354485 | 1 | 0.800851 | –0.84688 | –0.70949 | –0.14989 | –0.00517 |
| Coast dist. | –0.43813 | 0.218259 | 0.395688 | 0.800851 | 1 | –0.79097 | –0.66678 | 0.088094 | –0.01271 |
| Temp. sum | 0.418602 | –0.07446 | –0.41492 | –0.84688 | –0.79097 | 1 | 0.305601 | –0.39451 | 0.042062 |
| Coordinate E/W | 0.279088 | –0.22084 | –0.18979 | –0.70949 | –0.66678 | 0.305601 | 1 | 0.661445 | –0.05927 |
| Coordinate N/S | –0.08671 | –0.09351 | 0.168581 | –0.14989 | 0.088094 | –0.39451 | 0.661445 | 1 | –0.07265 |
| Stoniness | 0.158614 | –0.01117 | –0.15065 | –0.00517 | –0.01271 | 0.042062 | –0.05927 | –0.07265 | 1 |
Proportion of attacked seedlings ranged from 0to 78% with a mean of 12% and a median of 7%. Estimates of these statistics within plots therefore had mean confidence limits of ±6%, and confidence limits extended to approximately ±11% for estimates close to 50% (Fig. 2). The percentage of seedlings killed by the pine weevil ranged from 0to 74% with a mean of 8% and a median of 3%.
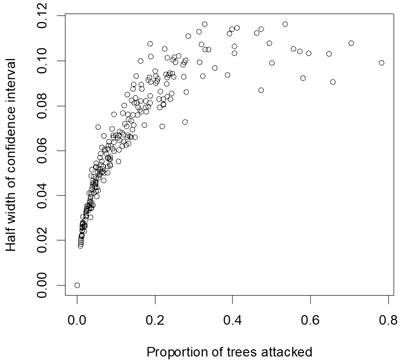
Fig. 2. Half width of confidence interval (α = 0.05) for estimates of proportion of seedlings attacked by pine weevils.
A stable and apparently optimal multi-linear regression model to represent proportion of seedlings attacked is shown in Table 4. The adjusted r2 value of the multi-linear regression model was 0.28 and the standard error was 0.12. Tree species was not significant in the model (P = 0.931). A plot of residuals versus fitted values for the model showed that it was reasonably unbiased (Fig. 3).
| Table 4. Regression model with proportion of seedlings attacked (λ = 0.39) as the dependent variable. If a λ-value is shown then this indicates the variable was subjected to a scaled power transformation. All interactions were made with transformed values. AIC = 349. Probabilities in bold are statistically significant. | ||||
| Variable | λ-value | Coefficient | Standard error | P-value |
| Intercept | 2.308e+04 | 1.477e+04 | 0.119299 | |
| Clear-cut age | 0.30 | –3.583e–01 | 1.387e–01 | 0.010276 |
| Proportion mineral soil | 0.70 | 3.740e–02 | 2.248e–02 | 0.097375 |
| Temperature sum | 1.49 | –2.110e+00 | 7.051e–01 | 0.003013 |
| Latitude | –2.00 | –4.618e+04 | 2.955e+04 | 0.119253 |
| Clear-cut age × Mineral soil | 1.824e–02 | 8.515e–03 | 0.033027 | |
| Mineral soil × Temp. sum | 6.809e–02 | 1.860e–02 | 0.000299 | |
| Latitude × Temp. sum | 4.221e+00 | 1.411e+00 | 0.003011 | |
| Mineral soil × Latitude × Temp. sum | –1.362e–01 | 3.720e–02 | 0.000299 | |
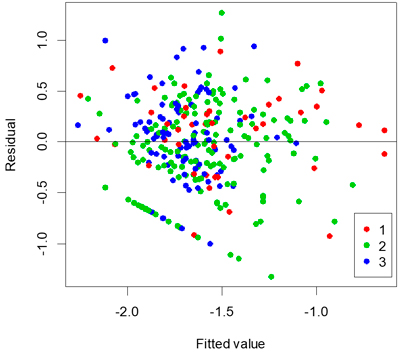
Fig. 3. Residuals versus fitted values coloured by age of clear-cut.
Adding calendar year as a random effect did not improve the fit (AIC = 402 versus AIC = 349), indicating that the year of sampling had little effect on variation in proportion of seedlings attacked.
The three most important variables in the model were percentage of planting spots with pure mineral soil on the surface, clear-cut age, and temperature sum. The model could have been constructed with either distance from the coast or elevation instead of temperature sum without substantially affecting the goodness of fit. The effects of mineral soil, clear-cut age and temperature sum interacted in the optimal model, as shown graphically in Fig. 4. Interactive effects that included latitude slightly changed the shape of the relationship and could have been left out the model without unduly affecting the principal findings, but the fit would have been poorer (AIC = 373 versus AIC = 349). When the model is applied to northern Sweden, the effects of temperature sum, percentage of seedlings in pure mineral soil and clear-cut age are depicted in a series of maps (Fig. 5).
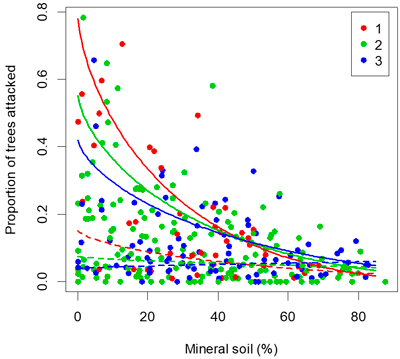
Fig. 4. Proportion of seedlings attacked by pine weevils versus proportion of seedlings planted in pure mineral soil. Lines show the fit of the multi-linear regression. The maximum temperature sum (1267 degree-days) is shown in full lines and the 25th percentile (909 degree-days) is shown with dashed lines. The colour of each point indicates the number of years since clearfelling.
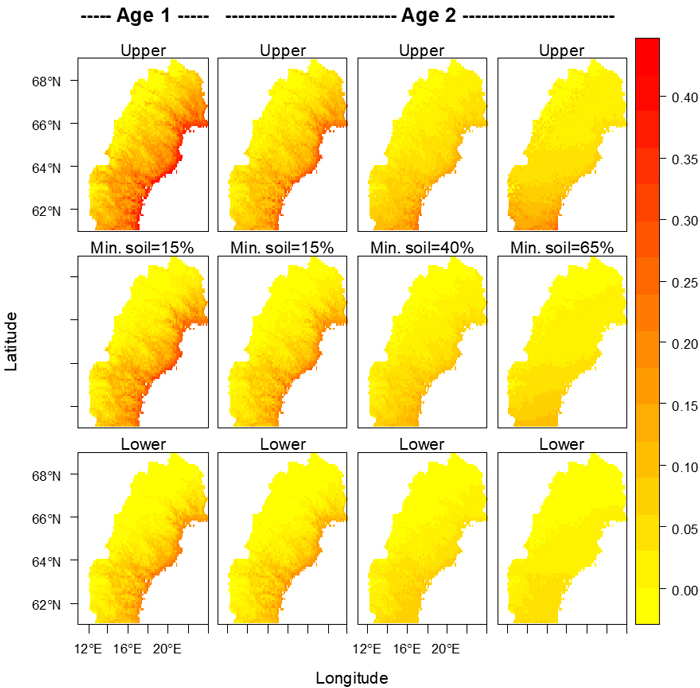
Fig. 5. Proportions of seedlings attacked by pine weevils for clear-cut ages 1 and 2 for several percentages (shown above each column of maps) of seedlings with mineral soil around their bases. The middle row shows the model’s expected values, and the upper and lower 95% confidence limits are shown in the upper and lower rows respectively. Maps were created using R (R Core Development Team 2004). Alpine areas devoid of trees were assumed to be those areas with a mean temperature between May 1st and September 30th less than 7.5 °C (Körner 1998), and in all maps the risk in those areas was set to zero.
Stoniness of the ground might have affected the proportion of seedlings attacked because residuals of the model were slightly correlated with that variable (r = 0.13), but as 72 records were missing an assessment of stoniness it was not included. Type of site preparation, tree species and site index were not significant when tested as independent variables.
A model of weevil attack calculated with only the more balanced 2 and 3 year old clear-cuts produced the same form of model as one that also included data from 1 year old clear-cuts. However, the reduced numbers of sites and a smaller range of the effect meant that terms including clear-cut age were not statistically significant. With these terms left in, a plot of this model against the proportion of seedlings in pure mineral soil by time since clearfelling almost perfectly overlaid an equivalent plot of the model that included 1 year old clear-cuts.
A logistic model of probability of attack combined with a model of proportion of attacked seedlings on sites with non-zero attack had a larger residual mean square (4.38) than the optimal single model of weevil attack (4.08). The residual mean squares were calculated from untransformed proportions attacked.
The proportion of attacked seedlings that had died at the assessment after one season increased with decreasing proportion of seedlings surrounded by pure mineral soil (Table 5). Residuals of this model were relatively unbiased (Fig. 6).
| Table 5. Regression model with proportion of attacked seedlings that died (λ = 1.275) as the dependent variable. If a λ-value is shown then this indicates the variable was subjected to a scaled power transformation. All interactions were made with transformed values. “% in mineral soil” is the percentage of seedlings at each site with a surround of mineral soil. | ||||
| Variable | λ-value | Coefficient | Standard error | P-value |
| Intercept | –0.189616 | 0.031980 | 1.61e–07 | |
| % in mineral soil | 0.70 | –0.012979 | 0.003085 | 8.76e–05 |
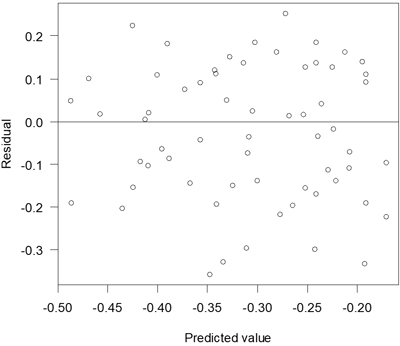
Fig. 6. Residuals versus predicted values for the model of death due to pine weevil attack.
For a subset of 27 sites an extra damage assessment was made also after the second season. These data were not included in the model but showed that seedling mortality caused by pine weevil increased with on average 53% during the second season as compared with the mortality after the first season (Fig. 7). The additional mortality by pine weevil the second season and mortality by other causes varied considerably between sites but there was still a general concordance between mortality due to pine weevil after one season and total mortality after two seasons.
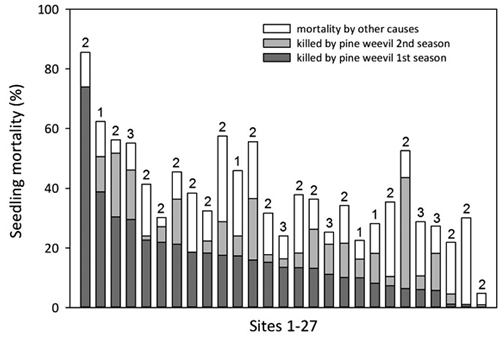
Fig. 7. Proportion of seedlings killed by pine weevil after one and two seasons, and total seedling mortality after two seasons on 27 sites (scattered from south to north but all within 100 km from the coast). Sites are arranged in decreasing order according to the proportion of seedlings killed by pine weevil the first season. Numbers above bars (1–3) denote the age of the clear-cut.
4 Discussion
4.1 Factors affecting damage risk
Our analysis of survey data from almost 300 reforestation areas in northern Sweden revealed that the risk of pine weevil attack on conifer seedlings can be predicted approximatively with the following three variables as the most important in the optimal model: the proportion of seedlings expected to be planted in mineral soil, age of clear-cut at the time of planting, and calculated temperature sum at the location. In particular, the strong influence by temperature sum on damage level was previously not well documented.
The optimal model was relatively imprecise but model precision could be improved in future surveys if more seedlings per site are inspected and if sites could be chosen to provide more balance across potential independent variables. Note that roughly half the standard error of the model can be attributed to errors of raw estimates of the dependent variable (Fig. 2), and so increasing numbers of trees sampled at each location would improve the overall standard error. Including stem diameter of the seedlings might further improve the model (Örlander and Nilsson 1999; Wainhouse et al. 2009; Luoranen et al. 2017). However, stem diameter did not appear to have a strong effect on weevil-induced seedling mortality in northern Sweden within the range of seedling sizes used in plantations (Johansson et al. 2015). A number of other site characteristics were tested as independent variables in the model but did not show any significant effects, and in this respect the results are in accordance with previous studies investigating risks for pine weevil damage (Wilson et al. 1996; Luoranen et al. 2017).
Three different species of conifer seedlings were included in the survey, Norway spruce, Scots pine and lodgepole pine, but species had no effect on damage in the model. This is in accordance with the results of a field experiment by Wallertz et al. (2014), where these three species did not differ significantly in attack rate after one season, although such differences were found for other conifer species in their comparison. Laboratory tests have shown differences in preference with regard to species but such differences do not always translate into corresponding differences in damage under field conditions (Zas et al. 2011). Moreover, plantations are usually monocultures without any choice available between different species of planted seedlings, which may erase the effect on damage by moderately large differences in preference.
Year of sampling had no detectable effect on likelihood of pine weevil attack, which is compatible with the view that pine weevil populations in forests managed by clear-cutting fluctuate relatively little between years (Björkman et al. 2015). This is because the population size is determined by the amount of breeding material (fresh stump roots) and forestry produce similar amounts in the landscape from year to year (Eidmann 1977; Nordlander et al. 2011; Skogsstyrelsen 2015). A relatively stable population size between years enables us to predict damage risks without any necessity to include additional information on population levels.
4.2 Effects of temperature and climate change
Impacts of temperature sum, distance from the coast or elevation all suggest an effect of temperature on pine weevil damage, and so it is appropriate to adopt the model with temperature sum even though the fits of the three alternative models were not significantly different. Temperature sum is also good to use in the model since it is a factor that can be adjusted to future climate scenarios by calculating a new prediction of temperature sum to represent the changed conditions.
Our results suggest that a warmer climate would result in increased damage by the pine weevil in major parts of northern Europe. However, there should not be any significant expansion in geographical distribution since H. abietis is already present almost everywhere in Europe where there is coniferous forest (Långström and Day 2004), from the Mediterranean (Zas et al. 2014; Semiz et al. 2017) up to the northernmost conifer stands of Norway, Sweden and Finland (Beijer-Petersen et al. 1962; Nilssen 1984). Nevertheless, major changes in geographical distribution with a warmer climate were predicted for H. abietis by Barredo et al. (2015), but this was a consequence of their reliance on data from the Global Biodiversity Information Facility (GBIF 2014) for the present distribution of this species, which did not include any occurrences in the warmer or cooler regions of Europe where H. abietis populations are actually present. The abundance of pine weevils within the present distribution range may, however, change significantly, for instance along elevational gradients in mountain areas (Chinellato et al. 2014) and towards higher latitudes in northern Europe.
In northern Sweden, a relatively high risk for damage caused by pine weevil can be expected along the coast of the Baltic Sea (Gulf of Bothnia) and at lower elevations up along the major river valleys, as illustrated in Fig. 5. With a warmer climate the risk will become considerable also at somewhat higher elevations, and thus on much wider areas than today. Moreover, in the areas with relatively high damage levels the risk may further increase towards the levels we see today in southern Sweden (Örlander and Nilsson 1999). All this points to a necessity for intensified pest management against the pine weevil in northern Sweden as the climate becomes warmer. For instance, physical protection of seedlings with a coating (Nordlander et al. 2009) is likely to be increasingly used in addition to site preparation and other measures currently keeping the damage at a low level (Nordlander et al. 2011).
4.3 Implications for forest management
The results corroborate previous findings that site preparation followed by planting in mineral soil is a most effective way of reducing damage by the pine weevil (Örlander and Nilsson 1999; Petersson and Örlander 2003; Nordlander et al. 2011). Site preparation serves to expose mineral soil and is therefore effective at reducing risk of weevil damage. In the survey of 292 planted clear-cuts, the highest attack rates (>40% of seedlings attacked) occurred only when the proportion of seedlings planted in mineral soil was lower than 40%, and when more than 60% of the seedlings were planted in mineral soil the attack rate was consistently well below 20% (Fig. 4). A high proportion of seedlings planted in mineral soil should generally give sufficient protection against pine weevil damage in the inland areas with lower damage risk.
The great positive effect on seedling survival by planting in pure mineral soil (Petersson et al. 2005) makes the quality of site preparation and subsequent choice of planting spots extremely important for successful forest regeneration. Wherever damage by pine weevil needs to be counteracted, the goal for site preparation on moraine soils should be to create suitable planting spots with pure mineral soil in high enough density but without affecting the ground too heavily (Nilsson et al. 2010). In northern Sweden disc-trenching and mounding are the predominant site preparation methods. A continuous method such as disc trenching causes a larger disturbance than patch-wise methods such as mounding (Bäcke et al. 1986). A major obstacle to obtaining high quality site preparation is the high degree of stoniness on Swedish clear-cuts (Örlander et al. 1990), and high quality planting spots are often not achieved (Nilsson et al. 2010) The current study indicates that the degree of stoniness may positively affect the frequency of pine weevil attack, which thus may result from a reduced quality of site preparation where there are many big stones. If site preparation techniques can be better adapted to local conditions, then quality will be improved and more seedlings survive. Even if the cost for site preparation becomes higher, such technical adaptations may prove to be cost-effective taking the total regeneration cost and final outcome of the regeneration efforts into account (Uotila et al. 2010).
The age of the clear-cut when planted is a significant factor for the risk of pine weevil attack and the general trend is that damage declines with increasing age, as shown by this and several previous studies (Örlander and Nilsson 1999; Nordlander et al. 2011). In commercial forestry, the intent is generally to replant clear-cuts as early as practical in order to avoid increased rotation length and problems with competing vegetation (Nilsson and Örlander 1999; Skogsstyrelsen 2016). In northern Sweden, however, the rotation length is already long and problems with competing vegetation are usually less severe than further south; thus the cost of having a fallow period of more than one season tends to be relatively low in northern Sweden. A fallow period of 2–3 years may therefore be kept as one of several useful tools in an integrated pest management strategy against pine weevil damage in northern Sweden.
Models can be imprecise and include a lot of variation but could still be very good as tools when decisions are to be made about the need for countermeasures against pine weevil damage. Maps of the kinds presented in this study indicate geographic variation in damage risks and this can together with other available information aid decisions about, for instance, areas where direct seedling protection with physical barriers is needed, where it should be enough with site preparation of standard or of highest quality, where a 3-year fallow period is an appropriate countermeasure, where sowing can be a good alternative instead of planting, and so on. The foresters know well how these measures work in their region from many different aspects, but they need the information on damage risk to make well adapted choices of countermeasures.
5 Conclusions
A multiple curvilinear model of the proportion of seedlings attacked by pine weevils at 292 sites in northern Sweden had a standard error of 0.12, and was relatively unbiased across the range of predictions. Pine weevil attack was significantly related to 1) the proportion of seedlings expected to be planted in mineral soil rather than soil covered with duff and debris, 2) age of clear-cut at the time of planting, 3) calculated temperature sum at each location, 4) latitude, which had a small effect only in interactions, and some other interactions between these variables.
Acknowledgements
This study was made possible by the kind cooperation of all forest owners that provided maps and other requested information about plantations to potentially include in the inventory (Holmen Skog AB, Stora Enso Skog AB, Sveaskog AB, SCA Skog AB, Persson Invest AB, and private forest owners affiliated to Norra skogsägarna AB). The study was part of the Swedish Hylobius Research Program financed by the Swedish forestry sector, and additional funding was received from the Forest Program of the Environmental Monitoring and Assessment at the Swedish University of Agricultural Sciences.
References
Akaike H. (1978). A Bayesian analysis of the minimum AIC procedure. Annals of the Institute of Statistical Mathematics 30(1): 9–14. https://doi.org/10.1007/BF02480194.
Ayres M.P., Lombardero M.J. (2000). Assessing consequences of global change for forest disturbance from herbivores and pathogens. The Science of the Total Environment 262(3): 263–286. https://doi.org/10.1016/S0048-9697(00)00528-3.
Bäcke J., Larsson M., Lundmark J.-E., Örlander G. (1986). Ståndortsanpassad markberedning – teoretisk analys av några markberedningsprinciper. Forskningsstiftelsen Skogsarbeten, Redogörelse No 3. 48 p. ISSN 0346-6671. [In Swedish].
Baffoe K.O., Dalin P., Nordlander G. Stenberg J.A. (2012). Importance of temperature for the performance and biocontrol efficiency of the parasitoid Perilitus brevicollis (Hymenoptera: Braconidae) on Salix. BioControl 57(5): 611–618. https://doi.org/10.1007/s10526-012-9443-5.
Bale J.S., Masters G.J., Hodkinson I.D., Awmack C., Bezemer T.M., Brown V.K., Butterfield J., Buse A., Coulson J.C., Farrar J., Good J.E.G., Harrington R., Hartley S., Jones T.H., Lindroth R.L., Press M.C. Symrnioudis I., Watt A.D., Whittaker J.B. (2002). Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology 8: 1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x.
Barredo J.I., Strona G., de Rigo D., Caudullo G., Stancanelli G., San-Miguel-Ayanz J. (2015). Assessing the potential distribution of insect pests: case studies on large pine weevil (Hylobius abietis L) and horse-chestnut leaf miner (Cameraria ohridella) under present and future climate conditions in European forests. EPPO Bulletin 45: 273–281. https://doi.org/10.1111/epp.12208.
Bärring L., Berlin M., Andersson Gull B. (2017). Tailored climate indices for climate-proofing operational forestry applications in Sweden and Finland. International Journal of Climatology 37(1): 123–142. https://doi.org/10.1002/joc.4691.
Bejer-Petersen B., Juutinen P., Kangas E., Bakke A., Butovitsch V., Eidmann H., Heqvist K.J., Lekander B. (1962). Studies on Hylobius abietis L. I. Development and life cycle in the Nordic countries. Acta Entomologica Fennica 17: 1–106.
Berggren Å., Björkman C., Bylund H., Ayres M.P. (2009). The distribution and abundance of animal populations in a climate of uncertainty. Oikos 118: 1121–1126. https://doi.org/10.1111/j.1600-0706.2009.17558.x.
Berlin M., Ericsson T., Andersson Gull B. (2014). Plantval – manual med implementeringsteknisk bakgrund. [Summary: Plantval – manual and background to technical implementation]. Arbetsrapport 851. Skogforsk, Uppsala, Sweden. 66 p. [In Swedish with English summary].
Björklund N., Nordlander G., Bylund H. (2003). Host-plant acceptance on mineral soil and humus by the pine weevil Hylobius abietis (L.). Agricultural and Forest Entomology 5: 61–65. https://doi.org/10.1046/j.1461-9563.2003.00163.x.
Björkman C., Bylund H., Nilsson U., Nordlander G., Schroeder L.M. (2015). Forest management to mitigate insect damage in a changing climate. p. 248–266. In: Björkman C., Niemelä P. (eds.). Climate change and insect pests. CABI, UK. 266 p + ix. ISBN 9781780643786.
Chinellato F., Faccoli M., Marini L., Battisti A. (2014). Distribution of bark and wood boring beetles along alpine elevational gradients. Agricultural and Forest Entomology 16: 111–118. https://doi.org/10.1111/afe.12040.
Cook R.D., Weisberg S. (1999). Applied regression including computing and graphics. John Wiley and Sons. New York. https://doi.org/10.1002/9780470316948.
Day K.R., Nordlander G., Halldórsson G. (2004). General biology and life cycles of bark weevils. Chapter 14. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht. p. 331–349. https://doi.org/10.1007/978-1-4020-2241-8_14.
Eidmann H.H. (1977). Recognition of the trophic environment of requisite-governed forest insects. Colloques Internationaux du CNRS 265: 151–163.
GBIF (2014). Global biodiversity information facility. https://www.gbif.org/.
Ge Z.M., Kellomäki S., Peltola H., Zhou X., Väisänen H., Strandman H. (2013). Impacts of climate change on primary production and carbon sequestration of boreal Norway spruce forests: Finland as a model. Climatic Change 118(2): 259–273. https://doi.org/10.1007/s10584-012-0607-1.
Giurca A., von Stedingk H. (2014). FSC pesticides policy in Sweden. Forest Stewardship Council, FSC Sweden. 16 p. https://se.fsc.org/rapporter.289.htm. [Cited 6 Oct 2014].
Hansen L.W., Ravn H.P., Geldmann J. (2005). Within- and between-stand distribution of attacks by pine weevil [Hylobius abietis (L.)]. Scandinavian Journal of Forest Research 20(2): 122–129. https://doi.org/10.1080/02827580510008284.
Inward D.J.G., Wainhouse D., Peace A. (2012). The effect on temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and potential impacts of climate change. Agricultural and Forest Entomology 14: 348–357. https://doi.org/10.1111/j.1461-9563.2012.00575.x.
Jactel H., Nicoll B.C., Branco M., Gonzalez-Olabarria J.R., Grodzki W., Långström B., Moreira F., Netherer S., Orazio C., Piou D., Santos H., Schelhaas M.J., Tojic K., Vodde F. (2009). The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science 66(7): 701. https://doi.org/10.1051/forest/2009054.
Jansson P.-E., Svensson M., Kleja D.B., Gustafsson D. (2008). Simulated climate change inpacts on fluxes of carbon in Norway spruce ecosystems along a climatic transect in Sweden. Biogeochemistry 89(1): 81–94. https://doi.org/10.1007/s10533-007-9147-6.
Johansson K., Hajek J., Sjölin O., Normark E. (2015). Early performance of Pinus sylvestris and Picea abies – a comparison between seedling size, species, and geographic location of the planting site. Scandinavian Journal of Forest Research 30(5): 388–400. https://doi.org/10.1080/02827581.2014.987808.
Keskitalo E.C.H., Bergh J., Felton A., Björkman C., Berlin M., Axelsson P., Ring E., Ågern A., Roberge J.-M., Klapwijk M.J., Boberg J. (2016). Adaptation to climate change in Swedish Forestry. Forests 7(2): 28. https://doi.org/10.3390/f7020028.
Kindvall O., Nordlander G., Nordenhem H. (2000). Movement behaviour of the pine weevil Hylobius abietis in relation to soil type: an arena experiment. Entomologia Experimentalis et Applicata 95: 53–61. https://doi.org/10.1046/j.1570-7458.2000.00641.x.
Kjellström E., Nikulin G., Hansson U., Strandberg G., Ullerstig A. (2011). 21st century changes in the European climate: uncertainties derived from an ensemble of regional climate model simulations. Tellus A: Dynamic Meteorology and Oceanography 63(1): 24–40. https://doi.org/10.1111/j.1600-0870.2010.00475.x.
Kolström M., Lindner M., Vilén T., Maroschek M., Seidl R., Lexer M.J., Netherer S., Kremer A., Delzon S., Barbati A., Marchetti M., Corona P. (2011). Reviewing the science and implementation of climate change adaptation measures in European forestry. Forests 2(4): 961–982. https://doi.org/10.3390/f2040961.
Körner C. (1998). A re-assessment of high elevation treeline positions and their explanation. Oecologia 115(4): 445–459. https://doi.org/10.1007/s004420050540.
Långström B. (1982). Abundance and seasonal activity of adult Hylobius-weevils in reforestation areas during first years following final felling. Communicationes Instituti Forestalis Fenniae 106. 23 p.
Långström B. (1985). Damage caused by Hylobius abietis in the years 1970–1971. Results from the Finnish part of a joint Nordic study. Folia Forestalia 612. 11 p. [In Finnish with English summary].
Långström B., Day K.R. (2004). Damage, control and management of weevil pests, especially Hylobius abietis. Chapter 19. In: Lieutier F., Day K.R., Battisti A., Grégoire J.-C., Evans H.F. (eds.). Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer Academic Publishers, Dordrecht. p. 415–444.
Lind P., Kjellström E. (2009). Temperature and precipitation changes in Sweden, a wide range of model-based projections for the 21st century. SMHI Reports Meteorology and Climatology No. 113. SMHI, Norrköping, Sweden. 50 p.
Lindner M., Maroschek M., Netherer S., Kremer A., Barbati A., Garcia-Gonzalo J., Seidl R., Delzon S., Corona P., Kolström M., Lexer M.J., Marchetti M. (2010). Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management 259(4): 698–709. https://doi.org/10.1016/j.foreco.2009.09.023.
Lindström A., Hellqvist C., Gyldberg B., Långström B., Mattsson A. (1986). Field performance of a protective collar against damage by Hylobius abietis. Scandinavian Journal of Forest Research 1(1–4): 3–15. https://doi.org/10.1080/02827588609382396.
Logan J.A., Régnière J., Powell J.A. (2003). Assessing the impacts of global warming on forest pest dynamics. Frontiers in Ecology and the Environment 1: 130–137. https://doi.org/10.1890/1540-9295(2003)001[0130:ATIOGW]2.0.CO;2.
Luoranen J., Viiri H. (2012). Soil preparation reduces pine weevil (Hylobius abietis (L.)) damage on both peatland and mineral soil sites one year after planting. Silva Fennica 46(1): 151–161. https://doi.org/10.14214/sf.71.
Luoranen J., Viiri H., Sianoja M., Poteri M., Lappi J. (2017). Predicting pine weevil risk: effects of site, planting spot and seedling level factors on weevil feeding and mortality of Norway spruce seedlings. Forest Ecology and Management 389: 260–271. https://doi.org/10.1016/j.foreco.2017.01.006.
Mattsson A. (2016). Reforestation challenges in Scandinavia. Reforesta 1: 67–85. https://doi.org/10.21750/REFOR.1.05.5.
Netherer S., Schopf A. (2010). Potential effects of climate change on insect herbivores in European forests – general aspects and the pine processionary moth as specific example. Forest Ecology and Management 259(4): 831–838. https://doi.org/10.1016/j.foreco.2009.07.034.
Nilssen A.C. (1984). Long-range arial dispersal of bark beetles and bark weevils (Coleoptera, Scolytidae and Curculionidae) in norhern Finland. Annales Entomologici Fennici 50: 37–42.
Nilsson U., Örlander G. (1999). Vegetation management on grass-dominated clearcuts planted with Norway spruce in southern Sweden. Canadian Journal of Forest Research 29(7): 1015–1026. https://doi.org/10.1139/x99-071.
Nilsson U., Luoranen J., Kolström T., Örlander G., Puttonen P. (2010) Reforestation with planting in northern Europe. Scandinavian Journal of Forest Research 25(4): 283–294. https://doi.org/10.1080/02827581.2010.498384.
Nordlander G., Bylund H., Örlander G.,Wallertz K. (2003). Pine weevil population density and damage to coniferous seedlings in a regeneration area with and without shelterwood. Scandinavian Journal of Forest Research 18(5): 438–448. https://doi.org/10.1080/02827580310001634.
Nordlander G., Bylund H., Björklund N. (2005) Soil type and microtopography influencing feeding above and below ground by the pine weevil Hylobius abietis. Agricultural and Forest Entomology 7: 107–113. https://doi.org/10.1111/j.1461-9555.2005.00257.x.
Nordlander G., Nordenhem H., Hellqvist C. (2009). A flexible sand coating (Conniflex) for the protection of conifer seedlings against damage by the pine weevil, Hylobius abietis. Agricultural and Forest Entomology 11: 91–100. https://doi.org/10.1111/j.1461-9563.2008.00413.x.
Nordlander G., Hellqvist C., Johansson K., Nordenhem H. (2011). Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. Forest Ecology and Management 262(12): 2354–2363. https://doi.org/10.1016/j.foreco.2011.08.033.
Nordlander G., Hellqvist C., Hjelm K. (2017). Replanting conifer seedlings after pine weevil emigration in spring decreases feeding damage and seedling mortality. Scandinavian Journal of Forest Research 32(1): 60–67. https://doi.org/10.1080/02827581.2016.1186220.
Odin H., Eriksson B, Perttu K. (1983). Temperaturklimatkartor för svenskt skogsbruk. Rapporter i skogsekologi och skoglig marklära Uppsala 45, Institutionen för Skoglig Marklära, SLU, Uppsala. 57 p. [In Swedish].
Örlander G., Nilsson U. (1999). Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scandinavian Journal of Forest Research 14(4): 341–354. https://doi.org/10.1080/02827589950152665.
Örlander G., Gemmel P., Hunt J. (1990). Site preparation – a Swedish overview. BC Ministry of Forests, FRDA Report 105. 61 p. https://www.for.gov.bc.ca/hfd/pubs/docs/Frr/Frr105.htm.
Petersson M., Örlander G. (2003). Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Canadian Journal of Forest Research 33(1): 64–73. https://doi.org/10.1139/x02-156.
Petersson M., Örlander G., Nordlander G. (2005). Soil features affecting damage to conifer seedlings by the pine weevil Hylobius abietis. Forestry 78(1): 83–92. https://doi.org/10.1093/forestry/cpi008.
R Development Core Team (2004). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/.
Selander J. (1993). Survival model for Pinus sylvestris seedlings at risk from Hylobius abietis. Scandinavian Journal of Forest Research 8(1–4): 66–72. https://doi.org/10.1080/02827589309382755.
Selander J., Immonen A., Raukko P. (1990). Resistance of naturally regenerated and nursery-raised Scots pine seedlings to the large pine weevil. Folia Forestalia 766. 19 p.
Semiz G., Erbilgin N., Holopainen J.K. (2017). Hylobius abietis L. feeding on the novel host Pinus brutia Ten. increases emission of volatile organic compounds. Journal of Applied Entomology 141: 133–140. https://doi.org/10.1111/jen.12310.
Skogsstyrelsen (2015). Bruttoavverkning 2015. [Summary: Gross felling in 2015]. Statistiska Meddelanden JO0312 SM 160. Statistiska centralbyrån, Stockholm. https://www.skogsstyrelsen.se/globalassets/statistik/statistiska-meddelanden/bruttoavverkning-jo0312/2015-bruttoavverkning-sm-jo0312.pdf.
Skogsstyrelsen (2016). Kunskapsplattform för skogsproduktion. Tillståndet i skogen, problem och tänkbara insatser och åtgärder. Meddelande 1/2016. [In Swedish]. https://shopcdn.textalk.se/shop/9098/art85/36356085-1bde4e-Kunskapsplattform_webb.pdf.
Skogsstyrelsen (2017). Produktion av skogsplantor 2016. [Summary: Production of seedlings 2016]. Statistiska Meddelanden JO0313 SM 1701. Statistiska centralbyrån, Stockholm. https://www.skogsstyrelsen.se/globalassets/statistik/statistiska-meddelanden/produktion-av-skogsplantor-jo0313/2016-statistiska-meddelanden-produktion-av-skogsplantor.pdf.
Solbreck C., Gyldberg B. (1979). Temporal flight pattern of the large pine weevil, Hylobius abietis L. (Coleoptera, Curculionidae), with special reference to weather. Zeitschrift für anbewandte Entomologie 88: 532–536. https://doi.org/10.1111/j.1439-0418.1979.tb02532.x.
Subramanian N., Bergh J., Johansson U., Nilsson U., Sallnäs O. (2016). Adaptation of forest management regimes in southern Sweden to increased risks associated with climate change. Forests 7(1): 8. https://doi.org/10.3390/f7010008.
Tan J.Y., Wainhouse D., Day K.R., Morgan G. (2010). Flight ability and reproductive development in newly-emerged pine weevil Hylobius abietis and the potential effects of climate change. Agricultural and Forest Entomology 12: 427–434. https://doi.org/10.1111/j.1461-9563.2010.00491.x.
Tylianakis J.M., Didham R.K., Bascomte J., Wardle D.A. (2008). Global change and species interactions in terrestrial ecosystems. Ecology Letters 11: 1351–13–63. https://doi.org/10.1111/j.1461-0248.2008.01250.x.
Uotila K., Rantala J., Saksa T., Harstela P. (2010). Effects of soil preparation method on economic result of Norway spruce regeneration chain. Silva Fennica 44(3): 511–524. https://doi.org/10.14214/sf.146.
Viiri H., Miettinen O. (2013). Feeding preferenses of Hylobius pinastri Gyll. Baltic Forestry 19: 161–164. https://www.balticforestry.mi.lt/bf/PDF_Articles/2013-19[1]/Viiri%20Heli.pdf.
von Sydow F. (1997). Abundance of pine weevils (Hylobius abietis) and damage to conifer seedlings in relation to silvicultural practices. Scandinavian Journal of Forest Research 12(2): 157–167. https://doi.org/10.1080/02827589709355397.
Wainhouse D., Staley J.T., Jinks R., Morgan G. (2009). Growth and defence in young pine and spruce and the expression of resistance to a stem-feeding weevil. Oecologia 158: 641–650. https://doi.org/10.1007/s00442-008-1173-0.
Wainhouse D., Inward D.J.G.,Morgan G. (2014). Modelling geographical variation in voltinism of Hylobius abietis under climate change and implications for management. Agricultural and Forest Entomology16: 136–146. https://doi.org/10.1111/afe.12043.
Wallertz K., Petersson M. (2011). Pine weevil damage to Norway spruce seedlings: effects of nutrient-loading, soil inversion and physical protection during seedling establishment. Agricultural and Forest Entomology 13: 413–421. https://doi.org/10.1111/j.1461-9563.2011.00536.x.
Wallertz K., Nordlander G., Örlander G. (2006). Feeding on roots in the humus layer by adult pine weevil, Hylobius abietis. Agricultural and Forest Entomology 8: 273–279. https://doi.org/10.1111/j.1461-9563.2006.00306.x.
Wallertz K., Nordenhem H., Nordlander G. (2014). Damage by the pine weevil Hylobius abietis to seedlings of two native and five introduced tree species in Sweden. Silva Fennica 48(4): article 1188. https://doi.org/10.14214/sf.1188.
Wallertz K., Hanssen K.H., Hjelm K., Floistad I.S. (2016). Effects of planting time on pine weevil (Hylobius abietis) damage to Norway spruce seedlings. Scandinavian Journal of Forest Research 31(3): 262–270. https://doi.org/10.1080/02827581.2015.1125523.
Wilson W.L., Day K.R., Hart E.A.(1996). Predicting the extent of damage to conifer seedlings by the pine weevil (Hylobius abietis L.): a preliminary risk model by multiple logistic regression. New Forests 12(3): 203–222. https://doi.org/10.1007/BF00027932.
Woollons R.C. (1998). Even-aged stand mortality estimation through a two-step regression process. Forest Ecology and Management 105(1–3): 189–195. https://doi.org/10.1016/S0378-1127(97)00279-X.
Zas R., Moreira X., Sampedro L. (2011). Tolerance and induced resistance in a native and an exotic pine species: relevant traits for invasion ecology. Journal of Ecology 99: 1316–1326. https://doi.org/10.1111/j.1365-2745.2011.01872.x.
Zas R., Björklund N., Nordlander G., Cendán C., Hellqvist C., Sampedro L. (2014). Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest, Hylobius abietis. Forest Ecology and Management 313: 212–223. https://doi.org/10.1016/j.foreco.2013.11.014.
Total of 77 references.

