Resistance of Scots pine half-sib families to Heterobasidion annosum in progeny field trials
Marčiulynas A., Sirgedaitė-Šėžienė V., Žemaitis P., Jansons Ā., Baliuckas V. (2020). Resistance of Scots pine half-sib families to Heterobasidion annosum in progeny field trials. Silva Fennica vol. 54 no. 4 article id 10276. https://doi.org/10.14214/sf.10276
Highlights
- Large genetic variation was estimated in the resistance of Scots pine half-sib families to root rot in field trials
- A strong relationship was observed between family resistance to root rot and phenolic compound concentration in the wood.
Abstract
Five Scots pine (Pinus sylvestris L.) progeny field trials, each established in different Lithuanian regions of provenance in 1983, were studied. Each progeny field trial consists of 140 half-sib families from seven populations (20 families from each population). The evaluation was carried out in 2012 and 2018 to assess the families resistance to Heterobasidion annosum (Fr.) Bref. An index of resistance in the infected plots was calculated. To verify the accuracy of the method, total phenolic compounds (TPC) was chosen as key parameter to compare with the plant resistance index. During the six years between the two assessments, the percentage of living Scots pine trees in the progeny field trials decreased up to 20 percentage points (range: 4 p.p. to 20 p.p.). In 2018 the area of H. annosum damaged plots (in percentage from total field trial area) varied from 17 to 27%. Tree mortality in the trial correlates with site soil fertility – more fertile soils were distinguished by higher tree percentage loss and vice versa. Using analysis from combined data of all progeny trials, the family variance component reached 13.3 ± 2.2% and family heritability was 0.81. Family heritability estimates for root rot resistance show possibilities of high breeding effectiveness. The correlations between the trials in family resistance estimates were negligible (ranging from 0 to 0.28). The significant high correlation coefficient was determined between the resistance index and TPC concentration (r = 0.77, p = 0.0003). This allows us to assume that plant resistance is directly linked on TPC synthesis. The results indicate that the chosen methods of chemical resistance for identification of root rot-resistant genotypes are applicable for the selection of Scots pine half-sib families in the field trials with higher resistance to pathogens.
Keywords
Pinus sylvestris;
field trials;
half-sib families;
root rot pathogen;
total phenolic compounds;
tree susceptibility
-
Marčiulynas,
Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, Liepų str. 1, LT-53101 Girionys, Kaunas District, Lithuania
E-mail
adas.marciulynas@mi.lt

- Sirgedaitė-Šėžienė, Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, Liepų str. 1, LT-53101 Girionys, Kaunas District, Lithuania E-mail vaida.seziene@mi.lt
- Žemaitis, Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, Liepų str. 1, LT-53101 Girionys, Kaunas District, Lithuania E-mail povilas.zemaitis@mi.lt
- Jansons, Latvian State Forest Research Institute “Silava”, Department of Forest Tree Breeding, Rigas St.t. 111, Salaspils LV-2169, Latvia E-mail aris.jansons@silava.lv
- Baliuckas, Institute of Forestry, Lithuanian Research Centre for Agriculture and Forestry, Liepų str. 1, LT-53101 Girionys, Kaunas District, Lithuania E-mail virgilijus.baliuckas@mi.lt
Received 22 November 2019 Accepted 24 August 2020 Published 3 September 2020
Views 89613
Available at https://doi.org/10.14214/sf.10276 | Download PDF

1 Introduction
Heterobasidion annosum (Fr.) Bref. is an important fungal pathogen of northern temperate and boreal forests, causing root rot and butt rot in conifers (Bendz-Hellgren et al. 1998). Economic losses due to root rot infection in Europe amount to hundreds of millions of Euros annually (Woodward et al. 1998). H. annosum leads to extensive mortality and is particularly destructive in young plantations established on dry, sandy acidic soils with low organic matter content, especially in stands occupying former arable land (Delatour et al. 1998; Fiodorov 1998; Lygis et al. 2004a). Spores of H. annosum infect fresh conifer stumps and wounds in growing conifers (primary infection). Mycelium of the fungus spreads along the roots and moves from tree to tree via root contacts (secondary spread; Kenigsvalde et al. 2016).
Primary infection initiates development of new disease centres in stands where infection was not previously present. Infection of the roots results in reduced growth rates and premature death (Hadfield et al. 1986). Often H. annosum infection in a stand results in open areas up to 0.5 ha (Lygis et al. 2004b), caused by Scots pine (Pinus sylvestris L.) death 4–5 years after the first thinning. Young Scots pine trees (up to 5–7 years old) dry out and die in 2–3 years, and in older trees the disease may last for 10–20 years.
There are several ways to protect a next-generation forest from H. annosum. Silvicultural actions, such as chemical, or biological control reduce opportunities for H. annosum to enter and spread within a stand (Vasiliauskas et al. 2004; Brandtberg et al. 1996). Another option that actively reduces the spread of H. annosum is to plant less susceptible tree genotypes. Significant differences in fungal sapwood colonization between different Norway spruce (Picea abies (L.) H. Karst.) and Scots pine genotypes were determined in experiments where these tree species were inoculated with H. annosum (Von Weissenberg 1975; Swedjemark and Karlsson 2004; Mukrimin et al. 2019). Skrøppa et al. (2015a) noted, that up to ~35% of this variation can be assigned to genotypic variation. Other host traits, such as growth capacity, growth rhythm, and wood density, according to inoculation experiments, could also be influenced by host genotype after fungal extension within the infected tree (Swedjemark and Karlsson 2006; Oliva et al. 2010; Zaluma et al. 2016; Marciulynas et al. 2019).
Tree resistance to pathogens is related, in addition to genotype, to the general viability of trees, their physiological processes, and their ability to synthesize and mobilize secondary metabolites (SM), usually phenolic compounds, in tissues as defensive compounds (Witzell and Martín 2008; Sallas et al. 2001; Marčiulynas et al. 2019). Low molecular weight phenolics may function as precursors for the synthesis of defensive compounds e.g. lignin (Bonello and Blodgett 2003), (Urbanek Krajnc et al. 2014), but they may also increase plant resistance via synergistic effects, or impact the pathogen due to variable concentration levels of phenolic compounds (Wallis et al. 2008; Edenius et al. 2012). Some authors indicate that stilbenes pinosylvin (PS) and pinosylvinmonomethylether (PSME), which occur in Scots pine heartwood, are involved in the resistance to fungal colonization and that they accumulate locally in the reaction zone (Lieutier et al. 1996; Bois and Lieutier 1997).
Variation in the concentration of different plant secondary metabolites (terpenes, phenolics, formylated phloroglucinol compounds, stilbenes, flavonoids, sideroxylonals) depends upon both genetic and environmental factors (Andrew et al. 2007; Külheim et al. 2011; Ganthaler et al. 2017a). Studies show that the ability of different genotypes to synthesize secondary metabolites and mobilize them against pathogens is variable, and therefore genetic selection is appropriate an appropriate method to select pathogen-resistant families (Thor and Stenlid 2004; Oliva et al. 2011). Several associations among phenotypic traits, for example wood properties (Beaulieu et al. 2011; Westbrook et al. 2013), growth and wood chemistry (Lepoittevin et al. 2012), and cold hardiness and bud set timing (Eckert et al. 2009; Holliday et al. 2010), have been reported in coniferous trees. However, associations have been only rarely reported for cellular phenotypes (e.g., metabolite concentrations) (Eckert et al. 2012) or disease resistance (Quesada et al. 2010).
This study was designed to develop a new method of application of chemical resistance (Total Phenolic Compounds (TPC) accumulation in trees) for identification of root rot-resistant genotypes and integrate the procedure into practical forestry. Five Scots pine progeny field trials were analysed to answer the following questions: (1) Can root rot damage evaluation in progeny field trials be successfully used in H. annosum resistant Scots pine genotype selection? (2) Does root rot resistance rate vary among different Scots pine genotypes? (3) Do the total phenolic compounds (TPC) of healthy trees correlate with Scots pine resistance against root rot?
2 Materials and methods
2.1 Progeny field trial description
In this study we used five Scots pine progeny field trials, established in different regions of Lithuanian provenance in 1983 (Table 1, Fig. 1). Each progeny field trial consists of 140 half-sib families from seven populations (20 families from each population). The same populations and families were planted in each field trial, for a total of 31 411 trees: 5030 to 7800 trees in each trial depending on the trial area. The same test design was used in all five field trials: full randomization in five to six replicates with ten trees in each replicate. All populations and families are represented in each replicate. Planting spacing in all the trials was 1.5 × 1.5 m, except in the Šilutė field trial, where spacing was 2.0 × 1.0 m. The average survival rate of the planted trees was 40% at age 36.
| Table 1. Characteristics of five Scots pine (Pinus sylvestris) progeny field trials, established in different regions of Lithuanian provenance in 1983. | ||||||
| Trial code * | Forest enterprise | Forest district | Area (ha) | Planting design (m) | Soil ** | Coordinates |
| 28PBZ008 | Veisiejai | Latežeris | 2.0 | 1.5 × 1.5 | Na | 53°58´N, 24°09´E |
| 03PBZ010 | Veisiejai | Kapčiamiestis | 2.5 | 1.5 × 1.5 | Nb | 53°58´N, 23°43´E |
| 06PBZ005 | Ignalina | Vaišniūnai | 1.5 | 1.5 × 1.5 | Na | 55°24´N, 26°09´E |
| 31PBZ007 | Nemenčinė | Purviniškės | 1.5 | 1.5 × 1.5 | Nc | 55°01´N, 25°38´E |
| 22PBZ009 | Šilutė | Žemaitkiemis | 1.5 | 2.0 × 1.0 | Nd | 55°14´N, 21°31´E |
| * The code in the Lithuanian Forest Seed Database. ** Na – very oligotrophic mineral soils of normal moisture; Nb – oligotrophic mineral soils of normal moisture; Nc – mesotrophic mineral soils of normal moisture; Nd – eutrophic mineral soils of normal moisture (Vaičys 2001). | ||||||
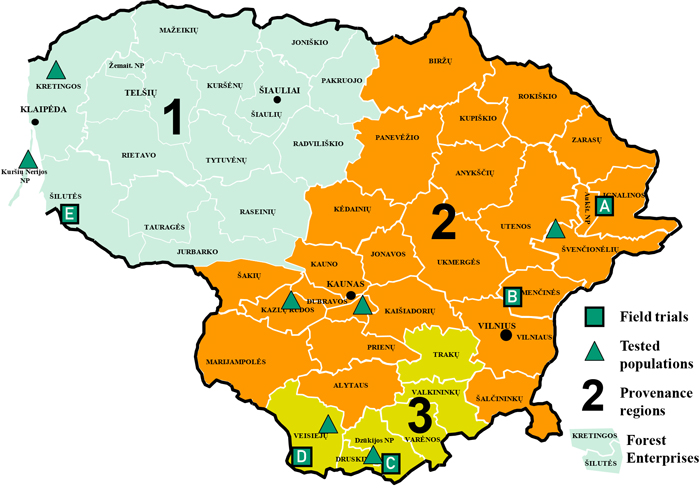
Fig. 1. Scots pine progeny field trials, provenance regions, and population origins. Scots pine progeny field trials used in the study: A – Ignalina, B – Nemenčinė, C – Druskininkai, D – Veisiejai, E – Šilutė. View larger in new window/tab.
2.2 Root rot damage evaluation
The evaluation of root rot damage in the same progeny field trials was carried out in 2012 and in 2018. The assessments were carried out at a six-year interval. The ages of the Scots pine trees in these progeny field trails were 30 years (2012) and 36 years (2018). The first step was to distinguish the root rot-damaged areas in the progeny field trials. Within these areas, root rot-damaged plots were defined according to groups of dead Scots pine trees caused by H. annosum (shown in Fig. 2). The cause of tree mortality in these plots was assessed by spot inspection of fallen tree roots and stems. In addition, H. annosum fruiting bodies was searched for in root rot-damaged plots in order to facilitate pathogen identification. If it was possible to find fruiting bodies of H. annosum on a dead scots pine trees, these damaged areas were classified as damaged by root-rot, if not – wood samples were taken from dead and live trees on the edges of the damaged area by drilling into the core at stump level and samples were taken to the laboratory for their identification. Individual pieces of the wood obtained were placed in separate sealed bags and incubated at 20 °C. After a three-week incubation period at room temperature, the samples were checked for mycelia, conidiophores, and conidia formation characteristic of H. annosum. If mycelium, conidiophores, and conidia formation characteristic of H. annosum were found on the wood samples, these trees were classified as damaged. If the H. annosum mycelium did not grown, then the trees were called healthy and the group of dead and living trees was not classified as root-rot damaged area.
An evaluation was carried out to assess Scots pine genotype resistance to H. annosum root rot by calculating an index of resistance at tree level in every root rot-damaged plot which was singled out as described below. The genotypic index of resistance was evaluated using root rot-damaged area in 2012 and 2018. The separation of infected plots was achieved at each field trial by fixing the borders of plots where the majority of trees were dead. 24 to 34 root rot-damaged plots were singled out in every trial in 2012, comprising approximately one third of the total trial area. The average rot-damaged plot size in 2012 was 139 m2 with the initial number of trees equal to 62. During the second assessment in 2018, only larger root rot-damaged plots were selected. In every trial in 2018, 10 to 17 plots were singled out as having root rot damage, consisting of approximately one-fifth of the total trial area. The average rot-damaged plot size in 2018 was 278 m2 with the initial number of trees equal to 124. Calculations were performed separately for the designated plots in the first assessment (2012) and the second assessment (2018) and a comparison of results was performed. The following is the formula of index of resistance (the obtained estimates that are summed up in the formula before were standardized to a mean of 0and a standard deviation of 1):
![]()
where dist is the inverted distance of any tree that survived in the plot to the plot centre (plot radius minus tree distance to the plot centre), rot is the ratio of number of trees in the plot to number of killed by H. annosum trees in the plot (the less trees survived the higher estimate), and rot_fam is the ratio of number of survived certain families’ trees (to which belong the evaluated tree) in the plot to the initial number of families’ trees in the delineated plot (the more family members survived the higher estimate). Distance from the centre of the plot was included in the formula in order to account for the fact that the trees situated closer to the centre were longer in the infected environment compared to the trees in the perimeter of the plot, so they are presumably more resistant (Rieksts-Riekstiņš et al. 2020).
2.3 Quantification of phenolic compounds
Wood samples of the 17 half-sib families (10 biological replicates from each) were collected in the Ignalina progeny field trial during the vegetation season in 2019 (total sample number: 17 Scots pine half-sib families × 10 biological replicates = 170 samples). Before analysis, the collected Scots pine wood samples were stored at −20 °C. For the preparation of methanolic extraction, 500 mg of fresh wood material was soaked in 10 ml of 75% methanol on a shaking table for 24 h at room temperature using a Kuhner Shaker X electronic shaker (Adolf Kühner AG, Birsfelden, Switzerland) and subsequently filtered through Whatman no. 1 filter paper.
The total phenolic content (TPC) of the extracts was evaluated using the Folin–Ciocalteu (FC) assay. 0.1mL of extract was then mixed with 0.1mL of 2 N FC reagent and 2.5mL of distilled water. After 6min, 0.5mL of 20% Na2CO3 was added and the mixture was incubated for 30 min. The absorbance was measured at 760nm using the T80 UV-VIS spectrophotometer (PG Instruments, Leicestershire, UK). The total phenolic content was expressed as chlorogenic acid equivalents in mg/100g of fresh material (CAE mg/ml).
2.4 Statistical analysis
The variance components were calculated with the following model using the SAS MIXED procedure (SAS Institute Inc. 2002–2012, version 9.4):
![]()
where µ is the grand mean, Ri is the fixed effect of replicate i, Pj is the fixed effect of population j, Fk is the random effect of family k, and Eijkl is the residual error. Standard errors of the estimates of variance components were found by Taylor expansions and the asymptotic covariance matrix of the estimates was obtained from MIXED procedure.
Family heritability ![]() was estimated as follows:
was estimated as follows:

where ![]() is the half-sib family variance component estimate from model (Eq. 2),
is the half-sib family variance component estimate from model (Eq. 2), ![]() is the residual variance estimate from model (Eq. 2), and n is the average number of trees per family in the corresponding trial. SAS CORR procedure was used for the Pearson correlation calculation.
is the residual variance estimate from model (Eq. 2), and n is the average number of trees per family in the corresponding trial. SAS CORR procedure was used for the Pearson correlation calculation.
3 Results
3.1 Damage to field trails
During the first field trial assessment in 2012, the average percentage of living Scots pine trees in progeny trials was about 50% of the initial planted number of trees (with the exception of Nemenčinė, where only 39% of trees were left) (Table 2). During the six years between the two assessments, the percentage of living trees decreased by a further 4 percentage points in the Druskininkai field trial, while in the Šilutė field trial it decreased by a further 20 percentage points. A decrease in tree percentage correlates with a soil fertility gradient – more fertile soils were distinguished by higher tree percentage loss and vice versa. In 2018, root rot-damaged areas in the analysed field trials varied from a 16.8% to a 27.1% of total field trial area. In our estimations, 1/3 to 1/2 of tree mortality was due to root rot damage, while the rest of the damage was due to self-thinning. The largest damaged sites were found in the Veisiejai progeny field trial (Table 2), where the damaged area is distinguished by its oval shape (Fig. 2D). The least-elongated sites were found in Druskininkai, while the most-elongated sites were found in Nemenčinė (where the average length of the site (y) was 4.7 times the width (x). It should be noted that no thinning were done in these trials until assessment in 2018.
| Table 2. Percentage of living scots pine trees in 2012 and 2018 years from the initial planted number of trees; the change of percentage points (∆) of living trees between the period of two assessments; the area of damaged plots in 2018 (expressed as percentage from total field trial area); and the average damaged plot size by Heterobasidion annosum in 2012 and 2018 (m2). | |||||||
| Code | Field trials | Percentage (%) | The change of percentage points (∆) | The area of damage plots in 2018 (%) | The average of damage plot in 2012 and 2018 (m2) | ||
| 2012 | 2018 | 2012 | 2018 | ||||
| A | Ignalina | 49 | 43 | –6 | 21.1 | 94 | 264 |
| B | Nemenčinė | 39 | 27 | –12 | 16.9 | 131 | 254 |
| C | Druskininkai | 56 | 52 | –4 | 16.9 | 92 | 282 |
| D | Veisiejai | 52 | 45 | –7 | 16.8 | 199 | 351 |
| E | Šilutė | 52 | 32 | –20 | 27.1 | 180 | 239 |

Fig. 2. Damaged areas in Scots pine progeny field trials during the 2012 and 2018 assessments. X – distance between the rows, Y – distance inside the rows. Scots pine progeny field trials used in the study: A – Ignalina, B – Nemenčinė, C – Druskininkai, D – Veisiejai trial, E – Šilutė. Damaged areas are contoured in black.
3.2 Scots pine families resistance to Heterobasidion annosum
Calculations are presented for the smaller (2012) and larger (2018) root rot-damaged plots in order to establish which method is more suitable for Scots pine resistance assessment. Two assessment comparisons are presented in Table 2. The estimates of the family component are large (and possibly overestimated) as the formula for calculation of resistance includes related components; e.g. the number of surviving trees in the plot and the number of surviving trees belonging to the same family as the evaluated tree.
Comparing family resistance estimates between smaller and larger damaged plots, the obtained correlation was 0.30. The most comparable results were found in the Veisiejai progeny field trial, while the biggest differences were found in Šilutė (where the root rot-caused thinning was the highest).
Correlations between breeding values of families showed that resistance was positively and significantly correlated with survival (0.18). Though family heritability estimates (Tables 3 and 4) show possible high breeding effectiveness for root rot resistance, the correlations between the trials in family resistance estimates were negligible (ranging from 0to 0.28). The problem could lie in spontaneous formation of damaged plots in the trials and in different combinations of families for evaluation occurring in the selected plots. Therefore, we have selected several combinations of families that occurred together in at least one designated root rot-damaged plot in each of the three field trials (Fig. 3). It can be seen that there is a tendency for families to preserve their level of resistance to root rot estimate in separate trials (e.g. No. 508, 482, 483, 495, 496, 492 and 493). This means that, despite the differences in infection rate, soil, and average root rot-damaged area size among the progeny field trials, there appears to be a genetic basis for Scots pine resistance to H. annosum.
| Table 3. Variance components and family heritability estimates of resistance to Heterobasidion annosum index in Lithuanian Scots pine progeny field trials (damaged plots in 2012). Variance components and standard errors given in percentage (* – 95%, ** – 99%, *** – 99.9%) | ||||||||
| Field trials | Family component (%) | ±SE | p-value | h2f | Population | Replicate | ||
| F criterion | p-value | F criterion | p-value | |||||
| Druskininkai | 54.4 | 10.5 | *** | 0.75 | 1.41 | . | 66.32 | *** |
| Ignalina | 18.6 | 4.4 | *** | 0.56 | 2.44 | * | 10.76 | *** |
| Nemenčinė | 23.2 | 5.0 | *** | 0.65 | 3.45 | ** | 1.71 | . |
| Šilutė | 50.7 | 10.2 | *** | 0.81 | 2.01 | . | 8.04 | *** |
| Veisiejai | 38.6 | 7.0 | *** | 0.77 | 0.93 | . | 4.91 | *** |
| Designation: h2f – family heritability. | ||||||||
| Table 4. Variance components and family heritability estimates of resistance to Heterobasidion annosum index in Lithuanian Scots pine progeny field trials (damaged plots in 2018). Variance components and standard errors given in percentage (* – 95%, ** – 99%, *** – 99.9%) | ||||||||
| Field trials | Family component (%) | ±SE | p-value | h2f | Population | Replicate | ||
| F criterion | p-value | F criterion | p-value | |||||
| Druskininkai | 53.4 | 11.0 | *** | 0.81 | 1.07 | . | 115.52 | *** |
| Ignalina | 62.0 | 11.3 | *** | 0.89 | 0.49 | . | 12.18 | *** |
| Nemenčinė | 48.5 | 8.6 | *** | 0.85 | 2.65 | * | 27.80 | *** |
| Šilutė | 26.1 | 4.7 | *** | 0.74 | 2.64 | * | 21.83 | *** |
| Veisiejai | 51.7 | 9.2 | *** | 0.82 | 1.56 | . | 10.50 | *** |
| Designation: h2f – family heritability. | ||||||||

Fig. 3. Estimate of resistance to Heterobasidion annosum of selected Scots pine half-sib families (with the same colouring) that occurred together in at least one designated root rot-damaged plot in each of the three selected field trials. Numbers represent the different Scots pine half-sib families.
The family component for Scots pine resistance was more significant when calculations were done on larger damaged plots. As is shown in Table 3 with the estimates from separate field trials, a slightly lower than in the rest of the trials family variance component was obtained in the Šilutė trial. Using analysis from all the field trials, the family variance component reached 13.3 ± 2.2% and the individual and family heritability estimates were 0.53 and 0.81, respectively. Although the population effect was not significant, the progeny of the populations of Juodkrantė and Labanoras were the most resistant to the spread of root rot. The population of Juodkrantė significantly differed from the populations of Darbėnai, Druskininkai, Dubrava, and Kazlų Rūda in resistance.
3.3 Total phenolic compounds
The most typical field trial site (Soil Na (Table 1)) for Scots pine as determined by site productivity in Lithuanian forests was selected Total Phenolic Compounds (TPC) accumulation in trees for identification of root rot-resistant genotypes (Fig. 4). The total phenolic compound (TPC) concentration was chosen as a key parameter to verify the plant resistance index. The secondary metabolites, such as phenolic compounds, are used as defensive compounds in Scots pine. The amount of TPC in Scots pine wood was determined during the growing season.
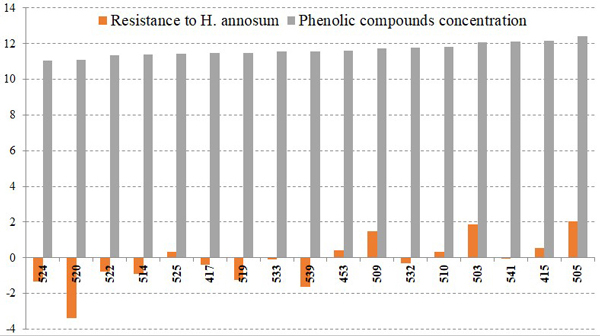
Fig. 4. Total phenolic compound concentration and resistance to Heterobasidion annosum estimates of 17 Scots pine half-sib families in the Ignalina field trial.
Our results showed that the half-sib families with a higher resistance index were characterized by higher TPC concentration (Fig. 4). A very low resistance index was determined in half-sib families No. 520 and 524, which also had low TPC concentration in the wood. There was a significant high correlation coefficient between the resistance index and TPC concentration (r = 0.77, p = 0.0003). This allows us to assume that plant resistance is directly linked to TPC synthesis.
4 Discussion
Five progeny field trials of Lithuanian Scots pine populations were evaluated for different levels of resistance of scots pine half-sib families to Heterobasidion annosum. The H. annosum damaged area results show that the most severe damage occurred in fertile habitats, which in our case was the Šilutė progeny field trial, where damaged trees with H. annosum accounted for more than 27% of total field trial area. Meanwhile, in less fertile habitats, the lesions were significantly lower (16.8% of the total field trial area). Such results confirm the data provided by other researchers indicating that the prevalence of root rot in stands is determined by the fertility of the site (Vasiliauskas 1964; Stenlid and Redfern 1998; Piri 2003; Vasiliauskas et al. 2005a).
Druskininkai and Šilutė experienced high levels of root rot spread despite the fact that they have not been thinned, which typically increases H. annosum spread. In many studies, greater stand age is reported as one of the factors contributing to a higher prevalence of infected trees, but all of our trials were the same age therefore this could not be causing the higher levels of prevalence in this study (Vasiliauskas 1964; Piri 2003; Pukkala et al. 2005; Vasiliauskas et al. 2005b). The same initial stand density was selected in all investigated progeny field trials, and therefore this factor could not affect inter-plot root rot incidence rate, as has been shown in other studies (Risbeth 1950; Thor et al. 2006; Žemaitis and Stakėnas 2016). However, a more detailed analysis of the lesions of H. annosum, shows that not all populations in the experimental plantations were affected equally. Therefore, it can be assumed from the results that Scots pine has resistance to H. annosum damage.
Our previous study results showed that a higher family effect is identified using Scots pine survival rates from root rot-damaged plots than those from the entire area of the field trial (Ministry of Environment research project report, “Assessment of Scots pine and Norway spruce genotypes for diversified breeding”, 2012–2013). Therefore, damaged plots were selected for this study. The family component of Scots pine resistance was more significant when calculations were carried out on larger damaged areas while eliminating small ones. The explanation could be that, more time is needed to form larger damaged area, resulting in longer exposure time for surviving trees. Therefore, only the most resistant genotypes avoids mortality due to H. annosum. It is known that high variability of susceptibility to root rot exists in natural populations of Scots pine (Marciulynas et al. 2019) and Norway spruce (Arnerup et al. 2010; Skrøppa et al. 2015a,b; Steffenrem et al. 2016), but it has been debated whether the genetic component is significant enough for resistance breeding. There is not much knowledge on Scots pine half-sib or sib family resistance index calculations using natural H. annosum incidence rate in progeny field trials. An inoculation study (Marciulynas et al. 2019) of Scots pine half-sib family resistance to root rot revealed that even progenies of trees which had survived in the damaged H. annosum plots differed significantly in resistance. Inoculation of Norway spruce families has shown that both genetic variation and heritability estimates are essential for breeding for resistance (Skrøppa et al. 2015a). The results did not support the strategy of tree selection in the affected plots with the aim to improve resistance in the progeny. On the other hand, the present study results confirm the possibility of using existing progeny trials with half-sib or sib families in breeding for resistance to root rot. Breeding for resistance to root rot does not reduce the breeding effectiveness of other traits (Ministry of Environment research project report, “Assessment of Scots pine and Norway spruce genotypes for diversified breeding”, 2012–2013).
Long-term experiments are useful for resistance studies, because root rot damage area increases with stand age and enables to determine damage plots for assessment family resistance to pathogen. The method of inoculation with pathogen is one of the most effective in screening resistant families or clones. However, there are numerous long-term experiments in European countries which could be used if proper methods were applied for evaluation of family resistance. Selected material could be used for the establishment of seed orchards and a diversified breeding strategy could be developed on this basis.
This study also focused on chemical resistance in different Scots pine families. The mortality differences of trees in the studies may be determined by different family susceptibilities to the pathogen (Swedjemark et al. 2001; Mukrimin et al. 2019) unequal pathogen virulence (Lakomy et al. 2011), incubation period (Stenlid and Swedjemark 1988; Zaluma et al. 2016), and plant defense mechanisms (i.e., secondary metabolites, phenolic compounds, terpenoid compounds) (Hammerschmidt 2005; Witzell and Martín 2008; Chong et al. 2009; Roach et al. 2014). The variation between different provenances and host genotype was reported for several stilbenes, flavonoids and terpenoids (Slimestad 1998; Evensen et al. 2000; Ganthaler et al. 2017b; Mukrimin et al. 2019). Our results indicate that the half-sib families with a higher resistance index are characterized by higher TPC concentration. A significant and high correlation coefficient was determined between the resistance index and TPC accumulation in Scots pine half-sib families. The findings of Mukrimin et al. (2019) revealed that Scots pine trees with low concentration of individual terpenoids (β-caryophyllene and α-humulene) were characterized by the greatest susceptibility to H. annosum necrosis. Data analysis of our research has shown the suitability of the chosen method of application of chemical resistance (Total Phenolic Content (TPC) accumulation in trees) for identification of root rot-resistant genotypes, which can be used as an additional indicator to determine resistance of trees to H. annosum.
It was found that by accounting for a smaller number and larger size of H. annosum damaged plots in the field trials compared to larger number and smaller size damaged plots would increase the efficiency of breeding for resistance to root rot. The efficiency of selection of resistant genotypes also increases with the age of field trials. The results indicate that a combination of methods may be directed towards the breeding of Scots pine half-sib families with higher resistance to pathogens. This could be used for forest management strategies, employed commercially for propagation and marketing, and used for afforestation.
Acknowledgments
This research is funded by the European Social Fund under the No 09.3.3 - LMT - K - 712 “Development of Competences of Scientists, other Researchers and Students through Practical Research Activities” measure.
References
Andrew R.L., Wallis I.R., Harwood C.E., Henson M., Foley W.J. (2007). Heritable variation in the foliar secondary metabolite sideroxylonal in Eucalyptus confers cross-resistance to herbivores. Oecologia 153: 891–901. https://doi.org/10.1007/s00442-007-0784-1.
Arnerup J., Swedjemark G., Elfstrand M., Karlsson B., Stenlid J. (2010). Variation in growth of Heterobasidion parviporum in a full-sib family of Picea abies. Scandinavian Journal of Forest Research 25(2): 106–110. https://doi.org/10.1080/02827581003730799.
Beaulieu J., Doerksen T., Boyle B., Clément S., Deslauriers M., Beauseigle S., Blais S., Poulin P.L., Lenz P., Caron S., Rigault P., Bicho P., Bousquet J., MacKay J. (2011). Association genetics of wood physical traits in the conifer white spruce and relationships with gene expression. Genetics 188(1): 197–214. https://doi.org/10.1534/genetics.110.125781.
Bendz-Hellgren M., Lipponen K., Solheim H., Thomsen I.M. (1998). Impact, control and management of Heterobasidion annosum root and butt rot in Europe and North America. The Nordic Countries. In: Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (eds.). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. p. 333–345.
Bois E., Lieutier F. (1997). Phenolic response of Scots pine clones to inoculation with Leptographium wingfieldii, a fungus associated with Tomicus piniperda. Plant Physiology Biochemistry 35: 819–825. https://doi.org/10.1023/A:1008624626399.
Bonello P., Blodgett J.T. (2003). Pinus nigra–Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiology Molecular Plant Pathology 63(5): 249–261. https://doi.org/10.1016/j.pmpp.2004.02.002.
Brandtberg P.O., Johansson M., Seeger P. (1996). Effects of season and urea treatment on infection of stumps of Picea abies by Heterobasidion annosum in stands on former arable land. Scandinavian Journal of Forest Research 11(1–4): 261–268. https://doi.org/10.1080/02827589609382935.
Budde K.B., Heuertz M., Hernández-Serrano A., Pausas J.G., Vendramin G.G., Verdú M., González-Martínez S.C. (2014). In situ genetic association for serotiny, a fire-related trait, in Mediterranean maritime pine (Pinus pinaster). New Phytologist 201(1): 230–241. https://doi.org/10.1111/nph.12483.
Chong J., Poutaraud A., Hugueney P. (2009). Metabolism and roles of stilbenes in plants. Plant Science 177(3): 143–155. https://doi.org/10.1016/j.plantsci.2009.05.012.
Cumbie W.P., Eckert A., Wegrzyn J., Whetten R., Neale D., Goldfarb B. (2011). Association genetics of carbon isotope discrimination, height and foliar nitrogen in a natural population of Pinus taeda L. Heredity 107: 105–114. https://doi.org/10.1038/hdy.2010.168.
Delatour C., von Weissenberg K., Dimitri L. (1998). Host resistance. In: Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (eds.). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. p. 143–166.
Dillon S.K., Nolan M., Li W., Bell C., Wu H.X., Southerton S.G. (2010). Allelic variation in cell wall candidate genes affecting solid wood properties in natural populations and land races of Pinus radiata. Genetics 185(4): 1477–1487. https://doi.org/10.1534/genetics.110.116582.
Eckert A.J., Bower A.D., Wegrzyn J.L., Pande B., Jermstad K.D., Krutovsky K.V., St Clair J.B., Neale D.B. (2009). Association genetics of coastal Douglas fir (Pseudotsuga menziesii var. menziesii, Pinaceae). I. Cold-hardiness related traits. Genetics 182(4): 1289–1304. https://doi.org/10.1534/genetics.109.102350.
Eckert A.J., Wegrzyn J.L., Cumbie W.P., Goldfarb B., Huber D.A., Tolstikov V., Fiehn O., Neale D.B. (2012). Association genetics of the loblolly pine (Pinus taeda, Pinaceae) metabolome. New Phytologist 193(4): 890–902. https://doi.org/10.1111/j.1469-8137.2011.03976.x.
Edenius L., Grzegorz M., Witzell J., Berghd J. (2012). Effects of repeated fertilization of young Norway spruce on foliar phenolics and arthropods: implications for insectivorous birds’ food resources. Forest Ecology of Management 277: 38–45. https://doi.org/10.1016/j.foreco.2012.04.021.
Evensen P.C., Solheim H., Hoiland K., Stenersen J. (2000). Induced resistance of Norway spruce, variation of phenolic compounds and their effects on fungal pathogen. Forest Pathology 30(2): 97–108. https://doi.org/10.1046/j.1439-0329.2000.00189.x.
Fiodorov N.I. (1998). Eastern Europe and Baltic countries. In: Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (eds.). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. p. 387–404.
Ganthaler A., Stöggl W., Kranner I., Mayr S. (2017a). Foliar phenolic compounds in Norway spruce with varying susceptibility to Chrysomyxa rhododendri: analyses of seasonal and infection-induced accumulation patterns. Front Plant Science 8 article 1173. https://doi.org/10.3389/fpls.2017.01173.
Ganthaler A., Stoggl W., Mayr S., Kranner I., Schuler S., Wischnitzki E. (2017b). Association genetics of phenolic needle compounds in Norway spruce with variable susceptibility to needle bladder rust. Plant Molecular Biology 94: 229–251. https://doi.org/10.1007/s11103-017-0589-5.
González-Martínez S.C., Wheeler N.C., Ersoz E., Dana Nelson C., Neale D.B. (2007). Association genetics in Pinus taeda L.I. Wood property traits. Genetics 175(1): 399–409. https://doi.org/10.1534/genetics.106.061127.
González-Martínez S.C., Huber D., Ersoz E., Davis J.M., Neale D.B. (2008). Association genetics in Pinus taeda L. II. Carbon isotope discrimination. Heredity 101: 19–26. https://doi.org/10.1038/hdy.2008.21.
Hadfield F.S., Goheen D.J., Filip G.M., Schmitt C.L., Harvey R.D. (1986). Root diseases in Washington and Oregon conifers. R6-FPM-250-86. USDA Forest Service, State and Private Forestry, Forest Pest Management, Portland, OR.
Hammerschmidt R. (2005). Phenols and plant-pathogen interactions: the saga continues. Physiology Molecular Plant Pathology 66(3): 77–78. https://doi.org/10.1016/j.pmpp.2005.08.001.
Hjältén J., Niemi L., Wennström A., Ericson L., Roininen H., Julkunen-Tiitto R. (2007). Variable responses of natural enemies to Salix triandra phenotypes with different secondary chemistry. Oikos 116(5): 751–758. https://doi.org/10.1111/j.0030-1299.2007.15365.x.
Holliday J.A., Ritland K., Aitken S.N. (2010). Widespread, ecologically relevant genetic markers developed from association mapping of climate related traits in Sitka spruce (Picea sitchensis). New Phytologist 188(2): 501–514. https://doi.org/10.1111/j.1469-8137.2010.03380.x.
Kenigsvalde K., Brauners I., Korhonen K., Zaļuma A., Mihailova A., Gaitnieks T. (2016). Evaluation of the biological control agent Rotstop in controlling the infection of spruce and pine stumps by Heterobasidion in Latvia. Scandianvian Journal of Forest Research 31(3): 254–261. https://doi.org/10.1080/02827581.2015.1085081.
Külheim C., Yeoh S.H., Wallis I.R., Laffan S., Moran G.F., Foley W.J. (2011). The molecular basis of quantitative variation in foliar secondary metabolites in Eucalyptus globulus. New Phytologist 191(4): 1041–1053. https://doi.org/10.1111/j.1469-8137.2011.03769.x.
Lakomy P., Kwasna H., Dalke-Swiderska M. (2011). The virulence of Heterobasidion parviporum population from Norway spruce stand in Suvalki forest district. Acta Scientiarum Polonorum Silvarum Colendarum Ratio et Industria Lignaria 10(3): 27–36.
Lepoittevin C., Harvengt L., Plomion C., Garnier-Géré P. (2012). Association mapping for growth, straightness and wood chemistry traits in the Pinus pinaster Aquitaine breeding population. Tree Genetics & Genomes 8: 113–126. https://doi.org/10.1007/s11295-011-0426-y.
Lieutier F., Sauvard D., Brignolas F., Picron V., Yart A., Bastien C., Jay-Allemand C. (1996). Changes in phenolics metabolites of Scots pine induced by Ophiostomabrunneo-ciliatum, a bark beetle associated fungus. Forest Pathology 26(3): 145–216. https://doi.org/10.1111/j.1439-0329.1996.tb00719.x.
Lygis V., Vasiliauskas R., Stenlid J., Vasiliauskas A. (2004a). Silvicultural and pathological evaluation of Scots pine afforestations mixed with deciduous trees to reduce the infections by Heterobasidion annosum s.s. Forest Ecology of Managament 201(2–3): 275–285. https://doi.org/10.1016/j.foreco.2004.07.013.
Lygis V., Vasiliauskas R., Stenlid J. (2004b). Planting Betula pendula on pine sites infested by Heterobasidion annosum: disease transfer, silvicultural evaluation, and community of wood-inhabiting fungi. Canadian Journal of Forest Research 34(1): 120 –130. https://doi.org/10.1139/x03-202.
Marčiulynas A., Sirgedaitė-Šėžienė V., Žemaitis P., Baliuckas V. (2019). The resistance of Scots pine (Pinus sylvestris L.) half-sib families to Heterobasidion annosum. Forests 10(3) article 287. https://doi.org/10.3390/f10030287.
Mukrimin M., Kovalchuk A., Ghimire R.P., Kivimäenpää M., Sun H., Holopainen J.K., Asiegbu F.O. (2019). Evaluation of potential genetic and chemical markers for Scots pine tolerance against Heterobasidion annosum infection. Planta 250: 1881–1895. https://doi.org/10.1007/s00425-019-03270-8.
Oliva J., Gonthier P., Stenlid J. (2010). Gene flow and inter‐sterility between allopatric and sympatric populations of Heterobasidion abietinum and H. parviporum in Europe. Forest Pathology 41(3): 243–252. https://doi.org/10.1080/02827581.2014.963144.
Oliva J., Bendz-Hellgren M., Stenlid J. (2011). Spread of Heterobasidion annosum s.s. and Heterobasidion parviporum in Picea abies 15 years after stump inoculation. FEMS Microbiology Ecology 75(3): 414–429. https://doi.org/10.1111/j.1574-6941.2010.01020.x.
Piri T. (2003). Silvicultural control of Heterobasidion root rot in Norway spruce forests in southern Finland. PhD thesis. The Fnnish Forest Research Institute, Research papers 898. 64 p. http://urn.fi/URN:ISBN:951-40-1887-7.
Pukkala T., Möykkynen T., Thor M., Rönnberg J., Stenlid J. (2005). Modeling infection and spread of Heterobasidion annosum in even-aged Fennoscandian conifer stands. Canadian Journal of Forest Research 35(1): 74–85. https://doi.org/10.1139/x04-150.
Quesada T., Gopal V., Cumbie W.P., Eckert A.J., Wegrzyn J.L., Neale D.B., Goldfarb B., Huber D.A., Casella G., Davis J.M. (2010). Association mapping of quantitative disease resistance in a natural population of loblolly pine (Pinus taeda L.). Genetics 186(2): 677–686. https://doi.org/10.1534/genetics.110.117549.
Redfern D.B., Pratt J.E., Hendry S.J., Low J.D. (2010). Development of a policy and strategy for controlling infection by Heterobasidion annosum in British forests: areview of supporting research. Forestry 83(2): 207–218. https://doi.org/10.1093/forestry/cpq005.
Rieksts-Riekstiņš R., Zeltiņš P., Baliuckas V., Brūna L., Zaļuma A., Kāpostiņš R. (2020). Pinus sylvestris breeding for resistance against natural infection of the fungus Heterobasidion annosum. Forests 11(1) article 23. https://doi.org/10.3390/f11010023.
Rishbeth J. (1950). Observations on the biology of Fomes annosus with particular reference to East Anglian pine plantations. The outbreaks of the disease and ecological status of the fungus. Annals of Botany 14(3): 365–383. https://doi.org/10.1093/oxfordjournals.aob.a083252.
Roach C.R., Hall D.E., Zerbe P., Bohlmann J. (2014). Plasticity and evolution of (+)-3-carene synthase and (−)-sabinene synthase functions of a Sitka spruce monoterpene synthase gene family associated with weevil resistance. Journal of Biological Chemistry 289(34): 23859–23869. https://doi.org/10.1074/jbc.M114.571703.
Sallas L., Kainulainen P., Utriainen J., Holopainen T., Holopainen J.K. (2001). The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine (Pinus sylvestris L.) seedlings. Global Change Biology 7(3): 303–311. https://doi.org/10.1046/j.1365-2486.2001.00408.x.
Singleton V.L., Orthofer R., Lamuela-Raventos R.M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 299: 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1.
Skrøppa T., Solheim H., Hietala A.M. (2015a). Variation in phloem resistance of Norway spruce clones and families to Heterobasidion parviporum and Ceratocystis polonica and its relationship to phenology and growth traits. Scandinavian Journal of Forest Research 30(2): 103–111. https://doi.org/10.1080/02827581.2014.963144.
Skrøppa T., Solheim H., Steffenrem A. (2015b). Genetic variation, inheritance patterns and parent–offspring relationships after artificial inoculations with Heterobasidion parviporum and Ceratocystis polonica in Norway spruce seed orchards and progeny tests. Silva Fennica 49(1) article 1191. https://doi.org/10.14214/sf.1191.
Slimestad R. (1998). Amount of flavonols and stilbenes during needle development of Picea abies; variation between provenances. Biochemical Systematics and Ecology 26(2): 225–238. https://doi.org/10.1016/S0305-1978(97)00099-9.
Steffenrem A., Solheim H., Skrøppa T. (2016). Genetic parameters for wood quality traits and resistance to the pathogens Heterobasidion parviporum and Endoconidiophora polonica in a Norway spruce breeding population. European Journal of Forest Research 135: 815–825. https://doi.org/10.1007/s10342-016-0975-6.
Stenlid J., Redfern D.B. (1998). Spread within the tree and stand. In: Woodward S., Stenlid J., Karjalainen R., Hüttermann A. (eds.). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. p. 125–141.
Stenlid J., Swedjemark G. (1988). Differential growth of S- and P-isolates of Heterobasidion annosum in Picea abies and Pinus sylvestris. Transactions of the British Mycological Society 90(2): 209–213. https://doi.org/10.1016/S0007-1536(88)80091-3.
Swedjemark G., Karlsson B. (2004). Genotypic variation in susceptibility following artificial Heterobasidion annosum inoculation of Picea abies clones in a 17-year-old field test. Scandinavian Journal of Forest Research 19(2): 103–111. https://doi.org/10.1080/02827580310018032.
Swedjemark G., Karlsson B. (2006). Mycelial growth and exclusion of Heterobasidion parviporum inoculated in branches of 15-year-old Picea abies clones. Scandinavian Journal of Forest Research 36(3): 209–214. https://doi.org/10.1111/j.1439-0329.2006.00452.x.
Swedjemark G., Johannesson H., Stenlid J. (1999). Intraspecific variation in Heterobasidion annosum for growth in sapwood of Picea abies and Pinus sylvestris. European Journal of Forest Pathology 29(4): 249–258. https://doi.org/10.1046/j.1439-0329.1999.00149.x.
Swedjemark G., Stenlid J., Karlsson B. (2001). Variation in growth of Heterobasidion annosum among clones of Picea abies incubated for different periods of time. Forest Pathology 31(3): 163–175. https://doi.org/10.1046/j.1439-0329.2001.00238.x.
Thor M., Stenlid J. (2004). Heterobasidion annosum infection of Picea abies following manual or mechanized stump treatment. Scandinavian Journal of Forest Research 20(2): 154–164. https://doi.org/10.1080/02827580510008338.
Thor M., Ståhl G., Stenlid J. (2006). Modelling root rot incidence in Sweden using tree, site and stand variables. Scandinavian Journal of Forest Research 20(2): 165–176. https://doi.org/10.1080/02827580510008347.
Urbanek Krajnc A., Novak M., Felicijan M., Kraševec N., Lešnik M., Zupanec N., Komel R. (2014). Antioxidative response patterns of Norway spruce bark to low-density Ceratocystis polonica inoculation. Trees 28: 1145–1160. https://doi.org/10.1007/s00468-014-1025-y.
Vaičys M. Miško dirvožemių klasifikacija (2001). In: Lietuvos Dirvožemiai. [Forest site types. Lithuanian soils]. Mokslas Publishers, Vilnius, Lithuania. p. 1040–1043. [In Lithuanian].
Vasiliauskas A. (1964). Šakninė pintis (Fomitopsis annosa (Fr.) Bond. Et Sing.) Lietuvos TSR eglynuose ir jos biologijos klausimai, susiję su panaudojimu kovos priemonių prieš ją. Daktaro disertacija. [Root rot (Fomitopsis annosa (Fr.) Bond. Et Sing.) in Lithuanian SSR spruce stands and its biology issues. PhD thesis]. Lietuvos miškų ūkio mokslinio tyrimo institutas, Kaunas. 220 p.
Vasiliauskas R., Lygis V., Thor M., Stenlid J. (2004). Impact of biological (Rotstop) and chemical (urea) treatments on fungal community structure in freshly Picea abies stumps. Biological Control 31(3): 405–413. https://doi.org/10.1016/j.biocontrol.2004.05.006.
Vasiliauskas R., Larsson E., Larsson K.H., Stenlid J. (2005a). Persistence and long-term impact of Rotstop biological control agent on mycodiversity in Picea abies stumps. Biological Control 32(2): 295–304. https://doi.org/10.1016/j.biocontrol.2004.10.008.
Vasiliauskas R., Lygis V., Larssson K.H., Stenlid J. (2005b). Airborne fungal colonisation of coarse woody debris in North Temperate Picea abies forest: impact of seasonal and local spatial scale. Mycological Research 109(4): 487–496. https://doi.org/10.1017/S0953756204002084.
Von Weissenberg K. (1975). Variation in relative resistance to spread of Fomes annosus in four clones of Picea abies. European Journal of Forest Pathology 5(2): 112–117. https://doi.org/10.1111/j.1439-0329.1975.tb00452.x.
Wallis C., Eyles A., Chorbadjian R., McSpadden Gardener B., Hansen R., Cipollini D., Herms D.A., Bonello P. (2008). Systemic induction of phloem secondary metabolism and its relationship to resistance to a canker pathogen in Austrian pine. New Phytologist 177(3): 767–778. https://doi.org/10.1111/j.1469-8137.2007.02307.x.
Westbrook J.W., Resende M.F.R., Muñoz P.D.R., Walker A.R., Wegrzyn J.L., Nelson Ch.D., Neale D.B., Kirst M., Huber D.A., Gezan S.A., Peter G.F., Davis J.M. (2013). Association genetics of oleoresin flow in loblolly pine: Discovering genes and predicting phenotype for improved resistance to bark beetles and bioenergy potential. New Phytologist 199(1): 89–100. https://doi.org/10.1111/nph.12240.
Witzell J., Martín J.A. (2008). Phenolic metabolites in the resistance of northern forest trees to pathogens – past experience sand future prospects. Canadian Journal of Forest Research 38(11): 2711–2727. https://doi.org/10.1139/X08-112.
Woodward S., Stenlid L., Karjalainen R., Hüttermann A. (1998). Heterobasidion annosum: biology, ecology, impact and control. CAB International, Wallingford, UK. 589 p.
Zaļuma A., Gailis A., Burņeviča N., Korhonen K., Gaitnieks T. (2016). Susceptibility of Picea abies and Pinus sylvestris Seedlings of Various origins to Heterobasidion annosum and H. parviporum. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences 70(1): 29–33. https://doi.org/10.1515/prolas-2016-0005.
Žemaitis P., Stakenas V. (2016). The main ecological factors influencing frequency of Norway spruce butt rot in mature stands in Lithuania. Russian Journal of Ecology 47(4): 355–363. https://doi.org/10.1134/S1067413616040172.
Total of 70 references.

